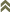Project Introduction
We present to you a taxonomic key for rapid identification using both molecular and morphological technology to identify thrips that have the potential to be introduced into the U.S. on imported plant material. This key was generated using Lucid v3.4 (CBIT, University of Queensland) diagnostic software and image manipulation for the compound microscope with Automontage (Syncroscopy, Cambridge) software. The key brings together color photographs of thrips taxonomic characters that are species specific and the results of internal transcribed spacer-restriction fragment length polymorphism (ITS-RFLP) as markers for individual thrips species (of any life stage) (Fig. 1).
 |
 |
Fig. 1: Frankliniella occidentalis: a) SEM picture of all ontogenetic stages of Western flower thrips, b) picture of a slide mounted adult female (bright field microscopy, (© GMoritz)
Lucid allows the builder of the key to coordinate photographs, ITS-RFLP results, character data, into an interactive, user-friendly identification key on a CD ROM.
The key provides biology for each thrips species which includes information about vectoring diseases, type of damage caused, and life cycle. The most important focus of this key is the molecular methods designed to rapidly (24 hours) identify thrips eggs, larvae or adults that are intercepted.
We anticipate this key to be used as a tool to aid inspection facilities in their task of ensuring our agriculture biosecurity in the U.S. This new tool will provide inspection agents with a taxonomic key that allows the user ease in manipulation of the key without scientific complication. Inspection agents who are not familiar with taxonomic keys will be able to navigate easily through this key.
Species list
In order to include thrips that have the potential to be introduced into the US on crop material we first listed the crops that are imported into the U.S. We used the USDA Department of Agricultural Research database to acquire this information and compiled a list of imported crops and the countries from which we import them. Secondly, we cross referenced the crops and the countries of import with thrips that are known pests of those imported crops in each country and added those thrips species to the list. We then included the thrips listed on the USDA reports identifying thrips that have been intercepted at ports of entry in the past ten years (Nickle 2003, 2004). Lastly we included thrips that are known as agricultural pests within the US to aid in determining between new introductions and established pests. The key includes 91 thrips species each with a description of their biology, country of origin, crop association, etc.
Anticipated benefit to the agriculture industry:The key will enable USDA-APHIS-PPQ agriculture inspection agencies, state and local growers and quarantine agents to rapidly identify pest thrips species intercepted at ports of entry. The agriculture industry will benefit by reducing losses caused by exotic thrips pests with early detection of thrips pests in imported crops. In addition, we are being proactive in protecting plant biosecurity by determining which exotic thrips pests should be kept from entering the United States. This information will prove useful as it is likely that requests for exemptions to Quarantine 37 will increase in future years.
We propose this novel key to be a supplement to morphology based identification for adult females. There are one or two morphological keys, limited to a few species, for larvae and no keys for thrips eggs. The rapid molecular method included in this key will provide accurate identification of both eggs and larvae. Molecular labs are more common throughout the United States and they can conduct this analysis providing results within 24 hrs. In addition, training workshops will be available to train inspectors on the use of the Lucid key as well as the molecular methods.
Project rationale
Agriculture crop sales in 2001 were estimated at $74.7 billion (USDA-NASS 2002). California and Florida are the two leading states in agricultural production and sales ($3.5 billion and $1.6 billion, respectively) and they are also among the top states in plant pest interceptions with 70% of all interceptions in the United States (USDA-NASS 2002). The Port of Los Angeles, CA intercepted 51.4% of its 2001 interceptions from four countries (Cost Rica, China, Thailand, and Guatemala) and the Port of Miami, FL intercepted 80% of its 2001 interceptions from Costa Rica and four other Central American countries.The cost of invasions is estimated at $137 billion in the US per year due to crop loss, cost of containment, pesticide applications, introduction of biological control agents and mechanical control (Anonymous 2002).
The continued relaxation of Quarantine 37, which allows the importation of plants established in growing media into the United States, will pose a potential threat to American growers in terms of the importation of exotic pests. The concern over invasive species is ever increasing in this country and there is growing fear of agricultural biological warfare. Rolstania solanacearum race 4 biovar 2 is listed by the USDA-Animal Plant Health Inspection Services (APHIS) as a bioterrorism agent. This bacterium was discovered on infected geranium cuttings in February 2002, imported from Kenya. The bacteria are also pathogenic to potatoes (USDA-NASS 2002).In 1999, APHIS monitored imports resulting in 2 million interceptions preventing importation of an estimated 53,000 plant pests (Anonymous 2002). Unfortunately, APHIS has the capacity to monitor only 2% of all cars, trucks, ships, and airplanes bringing products and people into the US. The Port Information Network (PIN) database reported that 14,878 arthropods were intercepted in 1990-1999. Twenty percent of all arthropods intercepted were not identified to taxonomic order due in part to damage, desiccation or were present only as eggs (Anonymous 2002).
While only 3.2% of all arthropod interceptions in the US were thrips, we believe that this is an underestimate due to thrips small size and their propensity to hide in tight places. Indeed, the rash of newly established thrips species in the US supports this contention. New thrips that have recently been ‘imported’ through worldwide movement of plant material include Scirtothrips perseae, Thrips palmi, Psydrothrips luteolus, and Holopothrips species. Scirtothrips perseae has been a serious pest on avocados in California since 1996 when large infestations caused damage to avocado fruits and foliage. It is thought that this species originated in Mexico and Central America (Nakahara 1997).
Thrips palmi, thought to originate from Southeast Asia, was discovered in Florida in 1991 when infestations were found damaging vegetable crops. Thrips palmi was subsequently discovered in the Netherlands and the Plant Protection Services there believed the source was Ficus plants from Florida. Today the Netherlands Plant Protection Services implements random inspections of Dutch Ficus growers and strict quarantine inspections on all imported Ficus (Parrella and Mound 1998). In 1996, Cuba accused the US of biological aggression by dropping large populations of Thrips palmi over their territory by airplane which subsequently created a ‘thrips plague’ on potato plantations in Cuba (CBW-Chronicle 1997). Psydrothrips luteolus was discovered on Dieffenbachia sp. in Honolulu Hawaii in 1991. This thrips caused serious damage to many Arecaceae plants and is thought to originate from Mexico (Nakahara 1994a).
Holopothrips sp., thought to be closely related to H. inquilinus, was discovered in south Florida in March 2002 damaging leaves of Caribbean trumpet tree, Tabebuia heterophylla and pink trumpet tree, T. pallida (FDOACS 2002). The state of Florida has listed 14 species of exotic Thysanoptera that have been introduced and established in Florida from 1987 to 1999 (Table 1) (Thomas 1999).
While many of these thrips species are primarily associated with vegetable crops, many can also damage agriculture crops and native plant species. Thrips palmi has been found on orchids and transmits tospoviruses in melon crops. Scirtothrips perseae has been primarily associated with avocados although there are numerous ornamental laurels and 2200 species of native laurels in California (Hickman 2003) that have the potential to be additional hosts for Scirtothrips perseae.
While one of us (C. O’Donnell) was conducting research on Thrips palmi as a potential pest of Ficus in Florida, two new thrips species were collected that were unknown to the US. Anascirtothrips arorai and Neohydatothrips geminus were collected on Ficus sp. in south Florida. Anascirtothrips arorai is from Panama and Taiwan and Neohydatothrips geminus is Neotropical. These two species feed on the leaves of Ficus sp. and Anascirtothrips arorai is well established in south Florida. The method by which they were introduced is unknown and no interception has been recorded for these species within the US (O'Donnell and Parrella 2000).With these invasions and the threat of agricultural bioterrorism increasing in the US, it is essential to produce new tools that will assist inspection agents with early detection, monitoring and identification of pests on imported plant material. Thrips and the viruses they transmit constitute one of the main pests of agricultural crops worldwide. Tomato spotted wilt virus, vectored solely by thrips, is estimated to cause more than $1 billion in damage to crops throughout the world annually (Flores 2003). Thrips are small in size and have cryptic habits making them amenable to movement in plant material. Thrips identification is problematic because they are transported around the world as eggs, larvae and adults (Fig. 1). Therefore when eggs and larvae are detected, plant material must be detained until the eggs and larvae are reared to the adult stage for identification (15-44 days).
Identification keys exist only for adult female thrips and current thrips identification keys available in the U.S. are dichotomous keys designed for the seasoned practitioner and do not deal with exotic thrips species. Many keys to identify adult species are out of date in that the keys were either developed many years ago e.g., Thrips of economic importance in California (Bailey 1938), The Thrips of California Part I: Suborder Terebrantia (Bailey 1957) or they do not make use of current technology and are region or genera specific e.g., The Genus Thrips Linnaeus (Thysanoptera: Thripidae) of the New World (Nakahara 1994b), Genus Frankliniella (Nakahara unpublished) and The thrips of Central and South America (Insecta: Thysanoptera) (Mound and Marullo 1996). The above keys are excellent literature for taxonomists and provide a great understanding about the species they include. However, for inspection agents these keys may be a large stumbling block for quick identification of the thrips that have been intercepted. We have produced an uncomplicated thrips key designed for growers and extension agents of North American Floriculture crops using color photographs and simple dichotomous couplets which is currently available for use on the world-wide-web (O'Donnell et al. 2003, Moritz 1994). Unfortunately, only adult female thrips are examined and the O´Donnell et al. (2003) key does not approach the identification of eggs and larvae. There is an urgent need for more rapid and accurate identification methods using molecular tools and newly emerging technology for all species and stages of potentially harmful thrips. Such keys targeting thrips as threats to agricultural security in Australia and in Germany have been developed (Moritz and Mound 2001, Moritz 2002, Moritz et al. 2001, 2004). Whether the USDA-APHIS can conduct adequate inspections, especially with an increase in the amount of plant material seems unrealistic without streamlining the tools with which they use to identify insects. In addition, there has been a dramatic decline in taxonomic expertise at the national and state levels over the past 20 years. Thrips are one example of small insects for which no new tools are available and there is limited national expertise*. Recommendations have been made in Predicting Invasions of Nonindigenous Plants and Plant Pests (Anonymous 2002) for USDA-APHIS to improve their technology in ‘detecting hitchhiking insects’ and to ‘synthesize the natural history of potential immigrant species’.
In order to limit the number of exotic thrips species introduced to the U.S. we must be able to quickly and accurately identify alien thrips species of potential economic importance. New tools are needed to identify those thrips that arrive on plant material from other countries. Keys developed for this purpose will assist USDA-APHIS, California Department of Food and Agriculture (CDFA), state inspectors and extension specialists. We must improve our methods of detecting new pests that threaten the national security of U.S. agriculture and our native ecosystems. Early detection and monitoring tools are essential for detecting pest thrips and the diseases they vector before they become symptomatic, spread, and become established.
* Sueo Nakahara is one of the foremost thrips taxonomists in the U.S. He worked at the Systematics Entomology Lab at USDA, Beltsville MD and retired in 1998. David Nickle an Isoptera and Orthoptera systematist has graciously filled in for Sueo Nakahara in the area of Thysanoptera identification at the Systematics Entomology Lab at USDA, Beltsville MD.
Table 1. Exotic Thysanoptera established in Florida (an excerpt from the list of exotic arthropod species established in Florida, FDOACS 2000)
Order Family
Organism
Year
Origin
County First Found
Thysanoptera
Thripidae
Bolacothrips striatopennatus (Schmutz)
1987
Asia
Hendry
Thysanoptera
Thripidae
Dendrothripoides innoxius (Karny)
1988
Asia
Palm Beach
Thysanoptera
Thripidae
Organothrips indicus Bhatti
1988
Asia
Hendry
Thysanoptera
Thripidae
Scirtothrips dorsalis Hood
1991
Asia
Okeechobee
Thysanoptera
Thripidae
Danothrips trifasciatus Sakimura
1992
Asia
Hendry
Thysanoptera
Thripidae
Neohydatothrips portoricensis (Morgan)
1992
Neotropical
Dade
Thysanoptera
Thripidae
Baileyothrips limbatus (Hood)
1993
Pacific
Palm Beach
Thysanoptera
Thripidae
Cheatanaphothrips leeuweni (Karny)
1993
Asia
Dade
Thysanoptera
Thripidae
Psydothrips luteolus Nakahara & Tsuda
1993
Pacific
Orange
Thysanoptera
Thripidae
Retithrips syriacus (Mayer)
1993
Africa
Broward
Thysanoptera
Thripidae
Elixothrips brevisetis (Bagnall)
1994
Asia
Broward
Thysanoptera
Thripidae
Asprothrips seminigricornis (Girault)
1995
Pacific
Orange
Thysanoptera
Aeolothripidae
Stomatothrips angustipennis Hood
1999
Neotropical
Hillsborough
Thysanoptera
Phlaeothripidae
Dolichothrips indicus (Hood)
1999
Asia
Pinellas
Table 2. Molecular lab available:
| Center for Functional Genomics | Phone: (518) 591-7200 |
| University at Albany | Office Fax: (518) 591-7201 |
| One Discovery Drive | Lab Fax: (518) 591-7211 |
| Rensselaer, NY 12144 | E-mail: genomics@albany.edu |
Table 3. Thysanoptera contacts (questions regarding visual and/or molecular identification).
| Name | Association |
|
Mark Hoddle |
University of California Riverside |
|
David Nickle |
USDA, Syst.- Entomology Laboratory |
|
Susan Broda |
USDA, APHIS, PPQ |
|
Cheryle O’Donnell |
USDA, APHIS, PPQ |
|
Gerald Moritz |
University of Halle |
|
Ray Gill |
CDFA (Retired) |
|
Laurence Mound |
CSIRO Australia |
|
Jitindra Bhatti |
University of Delhi, India |
|
Joeseph Funderburk |
University of Florida |
Lucid v3.4
The Lucid software has two functions i.e., a builder function and a player function. The builder function allows the author(s) of the key to design the key and enter both character states and taxa and then score each taxon for character state. This data is compiled in the builder function into a skeleton format with only taxa, characters, states, and scores inserted. Within the builder function we will access the ‘Taxon Properties’ menu and enter in the Auto-Montage images, ITS-RFLP gel images as well as the biology, damage to plant material, established locations, and diseases vectored for each species. Lucid compiles a comprehensive key formatted for a compact disk. The results render a comprehensive Thysanoptera key on CD ROM.
The Lucid player function allows an inspection agent to navigate through the key using character state couplets that segregate and delimit taxa with each character state choice. In the player function the user can select the ‘Best button’ which will prompt the character state that is most determinate of all the characters in the list. The player function allows the user to choose the method of navigation they would prefer or to initiate special program behavior, like "prune redundants" or "Auto Best". If the user select the "Best" button the key will prompt the character state that is most determinate of all characters in the list.
The importance of this work is to provide as many tools possible for thrips identification to all possible practitioners. Agriculture inspection agencies will also use the Lucid key as a tool to compare exotic thrips populations with North American populations first by using morphological features and second by using ITS-RFLP analyses.
The CD ROM was produced and disseminated through the University of Halle-Wittenberg, Germany, and the University of California. Copies have been distributed to the USDA-APHIS-PPQ, CDFA, and local inspection agencies, extension personnel who attended our training workshop on October 15-17, 2007 at UC Davis, Davis, California.The key includes the protocols for the ITS-RFLP analyses that are needed to identify eggs and larvae. We developed a network of technical experts to help answers technical questions, ensuring that the inspection agents can inquire about the technical function of the key. In addition, we provided a resource page listing Thysanoptera experts, and molecular labs that will help the users in problem solving i.e., a list of contact names, emails, addresses and phone numbers (Tables 2 and 3).
References and further reading
Anonymous (2002): Predicting invasions of non-indiginous plants and plant pests. National Academy Press: 1-185.
Araraki, N & Okajima, S (1998): Notes on the biology and morphology of a predatory thrips, Franklinothrips vespiformis (Crawford) (Thysanoptera: Aeolothripidae): first record from Japan. Entomological Science 1: 359–363.
Bailey, SF (1938): Thrips of economic importance in California. University of California College of Agriculture Agricultural Experiment Station Berkley, California Circular 346: 1-77.
Bailey, SF (1948): Grain and grass–infesting thrips. Journal of Economic Entomology 41: 701–706.
Bailey, SF (1957): The thrips of California Part I: Suborder Terebrantia. Bulletin of the California Insect Survey 4, no. 5: 143-220.
Bhatti, JS (1994): Phylogenetic relationships among Thysanoptera (Insecta) with particular reference to the families of the Order Tubulifera. Zoology (Journal of Pure and Applied Zoology) 4: 93–130.
Bhatti, JS (2006): The classification of Terebrantia (Insecta) into families. Oriental Insects 40: 339–375.
Butt, TM & Brownbridge, M (1997): Fungal pathogens of thrips. Pp. 399–434. In Lewis, T [ed.] Thrips as Crop Pests. Wallingford, UK: CABI. 740 pp.
CBW-Chronicle (1997): America accused of violating the biological and toxins weapons convention, Henry L. Stimson Center. CBW Chronicle.
Childers, CC (1997): Feeding and oviposition injuries to plants. Pp. 505–538. In Lewis, T [ed.] Thrips as Crop Pests. Wallingford, UK: CABI. 740 pp.
Chisholm, IF & Lewis, T (1984): A new look at thrips (Thysanoptera) mouthparts, their action and effects of feeding on plant tissue. Bulletin of Entomological Research 74: 663–675.
Cott, HE (1956): Systematics of the suborder Tubulifera (Thysanoptera) in California. Publications in Entomology, University of California 13: 1–216.
Crespi, BJ, Morris, DC & Mound, LA (2004): Evolution of ecological and behavioural diversity: Australian Acacia thrips as model organisms. Australian Biological Resources Study, Canberra & Australian National Insect Collection, Canberra. 328pp.
Diffie S, Edwards GB, & Mound, LA 2008. Thysanoptera of Southeastern U.S.A.: A checklist for Florida and Georgia. Zootaxa 1787: 45-62.
FDOACS (2000): List of exotic arthropod species established in Florida. Florida Department of Agriculture, Consumers Services.
FDOACS (2002): Pest alert Holopothrips sp. an introduction thrips pest of trumpet trees. Florida State University Department of Agricultural and Consumer Services.
Gordh, G & Headrick, D (2001): A dictionary of entomology. CABI Publishing, Oxon & New York, 1032 pp.
Gullan, PJ & Cranston, PS (1994): The Insects: An Outline of Entomology . Chapman and Hall, London. 491 pp.
Heming, BS (1970): Postembryonic development of the female reproductive system in Frankliniella fusca (Thripidae) and Haplothrips verbasci (Phlaeothripidae) (Thysanoptera). - Misc. Public. Entomol. Soc. Am. 7 (2): 199-234.
Heming, BS (1970): Postembryonic development of the male reproductive system in Frankliniella fusca (Thripidae) Misc. Public. Entomol. Soc. Am. 7 (2): 235-272.
Heming, BS (1971): Functional morphology of the thysanopteran pretarsus. - Canadian Journal of Zoology 49 (1): 91-108.
Heming, BS (1972): Functional morphology of the pretarsus in larval Thysanoptera. - Canad. J. Zool. 50: 751-766.
Heming, BS (1973): Metamorphosis of the pretarsus in Frankliniella fusca (HINDS) (Thripi-
dae) and Haplothrips verbasci (OSBORN) (Phlaeothripidae) (Thysanoptera). - Canadian Journal of Zoology 51 (12): 1211-1234.
Heming, BS (1975): Antennal structure and metamorphosis in Frankliniella fusca (HINDS) (Thripidae) and Haplothrips verbasci (OSBORN) (Phlaeothripidae) (Thysanoptera). - Quaest.
Entomol. 11: 25-68.
Heming, BS (1978): Structure and function of the mouthparts in larvae of Haplothrips verbasci (OSBORN) (Thysanoptera, Tubulifera, Phlaeothripidae). - J. Morph. 156: 1-38.
Heming, BS (1979): Origin and fate of germ cells in male and female embryos of Haplothrips verbasci (OSBORN) (Insecta, Thysanoptera, Phlaeothripidae). - J. Morph. 160: 323-344.
Heming, BS (1980): Development of the mouthparts in embryos of Haplothrips verbasci
(OSBORN) (Insecta, Thysanoptera, Phlaeothripidae). - J. Morph. 164: 235-263.
Heming, BS (1993): Structure, Function, ontogeny, and evolution of feeding in thrips (Thysanoptera). In: Schaefer CW & Leschen RAB (Editors), Functional morphology of insect feeding. - Entomological Society of America, Lanham, Maryland, San Antonio, Texas: 3-41.
Heming, B. S. (1995): History of germ line in male and female thrips. In: Parker, BL, Skinner, M & Lewis, T (Editors), Thrips Biology and Management. Series A: Life Sciences. - Plenum Press New York & London, Burlington, Vermont, pp. 505-535.
Heming, BS (2003): Insect development and evolution. Cornell University Press, Ithaca and London, 444pp.
Hickman, JC (2003): The Jepson manual: higher plants of California. University of California Press, Berkeley.
Hoddle MS, Mound LA & Nakahara S (2004): Thysanoptera recorded from California, USA: A checklist. Florida Entomologist 87: 317–323.
Hunter, WB & Ullman, DE (1992): Anatomy and ultrastructure of the piercing-sucking
mouthparts and paraglossal sensilla of Frankliniella occidentalis (PERGANDE) (Thysanoptera:
Thripidae). - Int. J. Insect Morphol. & Embryol. 21 (1): 17-35.
Hunter, WB & Ullman, DE (1994): Precibarial and cibarial chemosensilla in the Western flower thrips, Frankliniella occidentalis (PERGANDE) (Thysanoptera: Thripidae). - Int. J. Insect Morphol. & Embryol. 23 (2): 69-83.
Hunter, WB, Ullman, DE & Moore, A (1993): Electronic monitoring: characterizing the
feeding behaviour of the western flower thrips (Thysanoptera: Thripidae). In: Ellsbury, MM,
Backus, MM & Ullman, DE (Editors), History, development, and application of AC electronic insect feeding monitors. - Thomas Say Publications in Entomology: Proceedings. Entomological Society of America, Maryland: 73-85.
Jacobson, RJ (1997): Integrated pest management (IPM) in glasshouses. Pp. 639–666. In Lewis, T [ed.] Thrips as Crop Pests. Wallingford, UK: CABI. 740 pp.
Jacot-Guillarmod, CFJ (1970): Catalog of the Thysanoptera of the world (Part 1). Ann. Cape Prov. Mus. (Nat. Hist.) 7 (1), 1-216.
Jacot-Guillarmod, CFJ (1971): Catalog of the Thysanoptera of the world (Part 2). Ann. Cape Prov. Mus. (Nat. Hist.) 7 (2), 217-515.
Jacot-Guillarmod, CFJ (1974): Catalog of the Thysanoptera of the world (Part 3). Ann. Cape Prov. Mus. (Nat. Hist.) 7 (3), 517-976.
Jacot-Guillarmod, CFJ (1975): Catalog of the Thysanoptera of the world (Part 4). Ann. Cape Prov. Mus. (Nat. Hist.) 7 (4), 977-1255.
Jacot-Guillarmod, CFJ (1978): Catalog of the Thysanoptera of the world (Part 5). Ann. Cape Prov. Mus. (Nat. Hist.) 7 (5), 1256-1556.
Jacot-Guillarmod, CFJ (1979): Catalog of the Thysanoptera of the world (Part 6). Ann. Cape Prov. Mus. (Nat. Hist.) 7 (6), 1557-1724.
Jacot-Guillarmod, CFJ & Brothers, DJ (1986): Catalog of the Thysanoptera of the world (Part 7). Ann. Cape Prov. Mus. (Nat. Hist.) 17 (1), 1-93.
Gordh, G & Headrick, D (2001): A dictionary of entomology. CABI Publishing, CAB International, Wallingford, 1032 pp.
Gullan, PJ & Cranston, PS (1994): The insects: An outline of Entomology. Chapman and Hall, London, 491pp.
Kirk, WDJ (1984): Ecologically selective coloured traps. - Ecological Entomology 9: 35-41.
Kirk, WDJ (1984): Pollen-Feeding in thrips (Insecta: Thysanoptera). - J. Zool., Lond. 204: 107-117.
Kirk, WDJ (1985): Aggregation and mating of thrips in flowers of Calystegia sepium. - Ecological Entomology 10: 433-440.
Kirk, WDJ (1985): Effect of some floral scents on host finding by thrips (Insecta: Thyanoptera). - J. Chemical Ecology 11: 35-43.
Kirk, WDJ (1985): Egg-Hatching in thrips (Insecta: Thysanoptera). - J. Zool., Lond. 207: 181-190.
Kirk, WDJ (1985): Pollen-feeding and the host specificity and fecundity of flower thrips (Thysanoptera). - Ecological Entomology 10: 281-289.
Kirk, WDJ (1988): Thrips and pollination biology. In: Ananthakrishnan, TN & Raman, A (Editors), Dynamics of Insect-Plant-Interactions. Recent Advances and Future Trends. Oxford, IBH, New Delhi: 129-135.
Kirk, WDJ (1993): Thrips and pollen. - J. Pure and Applied Zoology 4: 251-258.
Kirk, WDJ (1994): The effects of density on the oviposition rate of flower thrips. - Courier Forschungsinstitut Senckenberg 178: 69-73.
Kirk, WDJ (1997): Feeding. Pp. 119–174. In Lewis, T [ed.] Thrips as Crop Pests. Wallingford, UK: CABI. 740 pp.
Kirk, WDJ (1997): Distribution, abundance and population dynamics. Pp. 217–258. In Lewis, T [ed.] Thrips as Crop Pests. Wallingford, UK: CABI. 740 pp.
Kirk, WDJ (2004): The link between cereal thrips and thunderstorms. - Acta Phytopathologica et Entomologica Hungarica 39 (1-3): 131-136.
Kirk, WDJ & Hamilton, JGC (2004): Evidence for a male-produced sex pheromone in the western flower thrips Frankliniella occidentalis. - J. Chemical Ecology 30 (1): 167-174.
Kirk WDJ & Terry I (2004): The spread of the western flower thrips Frankliniella occidentalis (Pergande). Agricultural and Forest Entomology 5: 301–310.
Lewis, T (1964): The weather and mass flights of Thysanoptera. - Ann. Appl. Biol. 53: 165-170.
Lewis, T (1973): Thrips their biology, ecology and importance importance. Academic Press London & New York 349pp.
Lewis, T (1997): Flight and dispersal. Pp. 175-196. In Lewis, T [ed.] Thrips as Crop Pests. Wallingford, UK: CABI. 740 pp.
Lewis, T (1997): Field and laboratory techniques. Pp. 435–476. In Lewis, T [ed.] Thrips as Crop Pests. Wallingford, UK: CABI. 740 pp.
Lewis, T (1997): Chemical control. Pp. 567–594. In Lewis, T [ed.] Thrips as Crop Pests. Wallingford, UK: CABI. 740 pp.
Lewis, T & Navas, DE (1962): Thysanopteran populations overwintering in hedge bottoms, grass litter, and bark. - Ann. Appl. Biol. 50: 299-311.
Loomans, AJM, Murai, T & Greene, ID (1997): Interactions with hymenopterous parasitoids and parasitic nematodes. Pp. 355–398. In Lewis, T [ed.] Thrips as Crop Pests. Wallingford, UK: CABI. 740 pp.
Moritz, G (1994): "Pictorial key to the economically important species of Thysanoptera in Central Europe". Bulletin OEPP/EPPO 24: 181-208.
Moritz, G (1995): Morphogenetic Development of some species of the order Thysanoptera
(Insecta). In: Parker, BL, Skinner, M & Lewis, T (Editors), Thrips biology and management. - Plenum Press & NATO Scientif. Affairs Division, New York and London: 489-504.
Moritz, G (1997): Structure, growth and development. Pp. 15-64. In Lewis, T [ed.] Thrips as Crop Pests. Wallingford, UK: CABI. 740 pp.
Moritz, G (2006): Thripse, Bd. 663, Westarp Wissenschaften, Hohenwarsleben, 384pp.
Moritz, G (2002): The biology of thrips is not the biology of their adults: a developmental
view. In: Marullo, R & Mound, LA (Editors), Thrips and Tospoviruses: Proc. 7th Int. Symp.
Thysanoptera. - Canberra: Aust. Natl. Insect Collect., Regio Calabria: 259-267.
Moritz, G, Delker, C, Paulsen, M, Mound, LA & Burgermeister, W (2000): Modern
methods for identification of Thysanoptera. - EPPO Bulletin 30: 591-593.
Moritz, G & Mound, LA (1995): CABIKEY to the common Thysanoptera of Europe. (13 MS DOS Discs). Wallingford, Oxon OX10 8DE, UK, CAB International.
Moritz, G & Mound, LA (2001): AQIS Identification guide (Thrips ID) Thysanoptera, species most likely to be imported into Australia on plant material. Canberra, Australian Quarantine and Inspection Service. CSIRO CD ROM.
Moritz G, Delker C, Paulsen M, Mound LA & Burgermeister W (2000): Modern methods for identification of Thysanoptera. OEPP/EPPO Bulletin 30: 591-593.
Moritz, G, Kumm, S & Mound, LA (2004): Tospovirus transmission depends on thrips ontogeny. Virus Research 100: 143-149.
Moritz G, Morris, DC & Mound, LA (2001): ThripsID- Pest thrips of the world. An interactive identification and information system. Melbourne, Aciar CD-ROM, CSIRO Publishing, Collingwood, Australia.
Moritz, G & Mound, LA (1999): AQIS Identification guide: Thysanoptera species most likely to be taken on plant material imported into Australia. - CDROM, Australian Quarantine and Inspection Service, Canberra.
Moritz G, Mound LA, Morris D & Goldarazena, A (2004): Pest thrips of the world - an identification and information system using molecular and microscopial methods. CBIT, University of Queensland, ISBN 1-86499-781-8.
Mound, LA (1983); Natural and disrupted patterns of geographical distribution in Thysanoptera (Insecta). Journal of Biogeography 10: 119–133.
Mound, LA (1997): Biological diversity. Pp. 197–216. In Lewis, T [ed.] Thrips as Crop Pests. Wallingford, UK: CABI. 740 pp.
Mound, LA & Marullo, R (1996): The thrips of Central and South America (Insecta: Thysanoptera). Memoirs on Entomology International 6: 1-487.
Mound, LA, Morison, GD, Pitkin, BR & Palmer, JM (1976): Handbooks for the identification of British insects-Thysanoptera 1(11):1-79. Royal Entomological Society of London.
Mound LA & Morris DC (2007): The insect order Thysanoptera: classification versus systematics. Pp 395–411, in Zhang ZQ & Shear WA [eds], Linnaeus Tercentenary: Progress in Invertebrate Taxonomy. Zootaxa 1668, 1–766.
Nakahara, S (1994): The Genus Thrips Linnaeus (Thysanoptera: Thripidae) of the New World. United States Department of Agriculture, Technical Bulletin Nr. 1822, 1-148.
Nickle, DA (2003): A checklist of commonly intercepted thrips (Thysanoptera) from Europe, the Mediterranean, and Africa at U.S. ports-of-entry (1983-1999). Part 1. Key to genera. Proceedings of the Entomological Society of Washington 105: 80-99.
Nickle, DA (2004): Commonly intercepted thrips (Thysanoptera) from Europe, the Mediterranean, and Africa at U.S. ports-of-entry. Part II. Frankliniella Karny and Iridothrips Priesner (Thripidae). Proceedings of the Entomological Society of Washington 106: 438-452.
O'Donnell, CA & Parrella, MP (2000): The biology and identification of selected North American Thysanoptera associated with ornamental plants., pp. 125, Entomology. University of California, Davis.
O'Donnell, CA, Mound, LA & Parrella, MP (2003): A multilevel identification key for thrips associated with flower crops in the US. University of California Davis, Entomology Department.
Okajima, S (2006): The Suborder Tubulifera (Thysanoptera). The Insects of Japan 2: 1–720. The Entomological Society of Japan, Touka Shobo Co. Ltd., Fukuoka.
Parker, BL & Skinner, M (1997): Integrated pest management (IPM) in tree crops. Pp. 615–638. In Lewis, T [ed.] Thrips as Crop Pests. Wallingford, UK: CABI. 740 pp.
Parrella, MP & Lewis, T (1997): Integrated pest management (IPM) in field crops. Pp. 595–614. In Lewis, T [ed.] Thrips as Crop Pests. Wallingford, UK: CABI. 740 pp.
Parrella, MP & Mound LA (1998): Making an example of Dutch ficus growers. Growers Talk.
Parrella, MP, Murphy, BC, O’Donnell, C & Casey C (2003): Insects and other animals, pp. 437-443. In Roberts, AV, Debener, T & Gudin, S [ed.], Encyclopedia of Rose Science. Elsevier Academic Press.
Priesner, H (1964): Ordnung Thysanoptera (Fransenfluegler, Thripse). in Franz, H, Bestimmungsbuecher zur Bodenfauna Europas 2: 1–242. Akademie–Verlag.
Sabelis, MW & van Rijn, PCJ (1997): Predation by insects and mites. Pp. 259–354. In Lewis, T [ed.] Thrips as Crop Pests. Wallingford, UK: CABI. 740 pp.
Schliephake, G & Klimt, KH (1979): Thysanoptera, Fransenfluegler. 66. Teil: Die Tierwelt Deutschlands. Fischer, Jena, 477pp.
Stannard, LJ (1968): The Thrips, or Thysanoptera, of Illinois. Bulletin of the Illinois Natural History Survey 29: 213–552.
Terry, LI (1997): Host selection, communication and reproductive behaviour. Pp. 65–118 in Lewis, T [ed.] Thrips as Crop Pests. Wallingford, UK: CABI. 740pp.
Tschuch G, Lindemann P, Niesen A, Csuk R, Moritz G (2005): A novel long chained acetate in the defensive secretion of thrips. Journal of Chemical Ecology 31 (7): 1555-1565.
Tschuch G, Lindemann P, Moritz G (2008): An unexpected mixture of substances in the defensive secretions of the Tubuliferan thrips, Callococcithrips fuscipennis (Moulton).Journal of Chemical Ecology 34 (6): 742-747.
Ullman, DE, Sherwood JL & German, TL (1997): Thrips as vectors of plant pathogens. Pp. 539–566. In Lewis, T [ed.] Thrips as Crop Pests. Wallingford, UK: CABI. 740 pp.
USDA-ASS (2002): Floriculture crops 2001 summary, USDA Cornell University. United States Department of Agriculture and National Agriculture Statistics Services.
USDA (2008): Summary of U. S. agricultural trade projections, fiscal years. In U. S. D. o. C. US Department of Agriculture and Bureau of Census [ed.]. USDA.
Wilson, TH (1975): A monograph of the subfamily Panchaetothripinae (Thysanoptera: Thripidae). Memoirs of the American Entomological Institute, Ann Arbor, Michigan, Nr.23, 354 pp.
Zur Strassen, R (2003): Die terebranten Thysanopteren Europas und des Mittelmeer–Gebietes. Die Tierwelt Deutschlands 74: 1–271.
Important web links:
Behaviour, sex allocation and molecular phylogenetics - Flinders University Adelaide
Complete Tospovirus Resource Page - Kansas State University & University of California
Pests and Diseases Image Library
Thrips Biology and Management - University of Florida
Thrips Biology and Pheromons - University of Keele
Thrips Biology, Development and Identification - University of Halle-Wittenberg
Thrips Collection of Entomological Museum - University of Alberta
Thrips Information and World Check List - CSIRO
Thrips Morphology Key - Key Point Graphics
Thrips of California