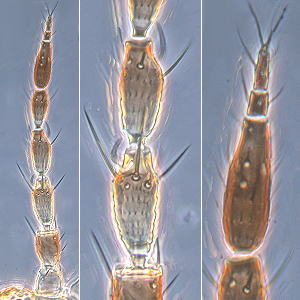
Frankliniella occidentalis (Pergande, 1895)
Thripinae, Thripidae, Terebrantia, Thysanoptera
Figures
Fig. 1: 8-segmented antenna, segments III and IV with forked sense cone, terminal segments VI-VIII
Fig. 2: Head dorsal with ocellar triangle
Fig. 3: Pronotum
Fig. 4: Meso- and metanotum
Fig. 5: Fore wing and fore wing distal region
Fig. 6: Tergite VIII with posteromarginal comb and tergite IX
Fig. 7: Tergite VIII, lateral region with stigma and ctenidia
Fig. 8: Tergites IX and X
Fig. 9 and 10: Female feeding on a bean leaf (Phaseolus vulgaris)
Introduction and recognition
Frankliniella occidentalis breeds on a wide range of plants, in flowers and on leaves, and causes serious crop losses both through feeding damage and as an important vector of tospoviruses. Both sexes fully winged; body color variable from yellow to brown, but widespread pest strain usually mainly dark yellow with brown areas medially on each tergite; antennal segments II & VI-VIII brown, III-V yellow with apices variably brown; legs mainly yellow washed with brown; fore wings pale with dark setae. Antennae 8-segmented; segments III & IV with forked sense cone, segment VIII longer, about 1.5 the length of antennal segment VII (Fig. 1). Head wider than long; 3 pairs of ocellar setae present, pair III about as long as distance between external margins of hind ocelli and arising on or just within anterior margins of ocellar triangle; postocular setae pair I present, pair IV longer than distance between hind ocelli (Fig. 2). Pronotum with 4-5 pairs of elongate setae (1 pair anteromarginally, 1 pair anteroangularly, 2 pairs posteroangularly and 1 pair of moderately elongate posteromarginal submedian setae) (Fig. 3). Mesofurca with spinula. Metanotal median area transverse at anterior and with irregular equiangular or longitudinal reticulations on posterior half; median setae longer than lateral setae and arising at anterior margin; campaniform sensilla present (Fig. 4). Mid and hind tarsi 2-segmented. Fore wing with 2 complete rows of veinal setae (Fig. 5). Tergites V-VIII with paired ctenidia laterally, ctenidia sometimes weakly developed on IV, on VIII anterolateral to spiracle (Fig. 7); posteromarginal comb on VIII complete, with short to moderately long microtrichia arising from triangular bases (Fig. 8). Sternites III-VII without discal setae; median setae of sternite VII arising at or close to posterior margin.
Male similar to female but smaller and paler; tergite VIII without marginal comb; IX with median pair of dorsal setae shorter than lateral pair; sternites III-VII with transverse glandular area.
Second instar larva white, antennae weakly shaded, tergite IX not with shaded transverse band extending just anterior to major setae; tergites with transverse rows of small, weakly developed, linear plaques, dorsal setae all blunt; tergite IX campaniform sensilla wide apart, almost anterior to setae S2, posterior margin with complete row of well-developed teeth.
Taxonomic identity
Species
Frankliniella occidentalis (Pergande, 1895)
Taxonomic history
Frankliniella helianthi Blunck & Neu, 1949
Frankliniella dahliae Moulton, 1948
Frankliniella dianthi Moulton, 1948
Frankliniella syringae Moulton, 1948
Frankliniella umbrosa Moulton, 1948
Frankliniella chrysanthemi Kurosawa, 1941
Frankliniella obscura Moulton, 1935
Frankliniella venusta Moulton, 1936
Frankliniella conspicua Moulton, 1936
Frankliniella occidentalis brunnescens Priesner, 1932
Frankliniella occidentlis dubia Priesner, 1932
Frankliniella claripennis Morgan, 1925
Frankliniella canadensis Morgan, 1925
Frankliniella trehernei Morgan, 1925
Frankliniella tritici maculata Priesner, 1925
Frankliniella moultoni Hood, 1924
Frankliniella nubila Treherne, 1924
Frankliniella tritici moultoni Hood, 1914
Euthrips helianthi Moulton, 1911
Euthrips tritici californicus Moulton, 1911
Euthrips occidentalis Pergande, 1895
Common name
Western flower thrips
Alfalfa thrips
Present taxonomic position
Family: Thripidae Stephens, 1829
Subfamily: Thripinae (Stephens) Karny, 1921
Genus: Frankliniella Karny, 1910
Genus description
The genus Frankliniella Karny, 1910
This genus is mainly known from the New World and contains about 230 species, many of them from the Neotropics (Mound & Marullo 1996, Cavalleri & Mound 2012). Some species are widely known as crop pests - Frankliniella occidentalis, Frankliniella intonsa, Frankliniella schultzei (all of them vectors of tospoviruses) and Frankliniella williamsi. The members in this genus are sometimes quite difficult to separate from one another and the classification has been in flux with many species later synonymized in association with color variations. They mostly have 3 pairs of well developed ocellar setae, 8-segmented antennae with segments III and IV having forked sense cones, usually 4-5 pairs of elongate pronotal setae (1 pair anteromarginally, 1 pair anteroangularly, 2 pairs posteroangularly, and 1 pair of moderately elongate posteromarginal submedian setae S2 which are longer than median seta S1), metanotal median setae arising at anterior margin, when present wings with complete rows of setae on the wing veins, paired ctenidia laterally on tergites V-VIII with those on VIII anterolateral to the spiracles, no discal setae on sternites, and the males are generally smaller and paler than the females (Mound & Marullo 1996; Stannard 1968).
Species description
Typical key character states of Frankliniella occidentalis
Coloration and body sculpture
Body color: mainly brown to dark brown or mainly pale to yellow, or with some darker markings
Surface of head, pronotum and fore legs: without obvious or with weakly reticulate sculpture
Antennae
Form of sense cones on antennal segments III and IV: emergent and forked on segments III and IV
Number of antennal segments: 8
Antennal segment I: without any setae on dorsal apical margin
Antennal segment II: without an exceptionally long seta at the inner apex
Antennal segment II shape: symmetric
Antennal segment III shape: symmetric
Shape of pedicel on antennal segment III: simple
Length of antennal segment III and IV: antennal segment III similar in length to segment IV
Forked sense cone on antennal segment IV: scarcely extending beyond base of segment V
Antennal segment IV and V: without a hyaline ring near the base
Antennal segment VI bears: not a remarkably dagger-shaped sensorium
Antennal segment VIII length: longer, about 1.5 the length of antennal segment VII
Head
Distance between bases of ocellar setae III: greater than width of first ocellus
Head: not prolonged in front of compound eyes
Pair of major postocular seta: longer than other postoculars and longer than distance between hind ocelli
Ocellar setae I: present
Length of ocellar setae II: shorter than setae III
Ocellar setae III: arising on anterior margin of, or in front of ocellar triangle or within ocellar triangle anterior to tangent of anterior margin of hind ocelli
Ocelli: present
Ocellar setae III length: about as long as distance between external margins of hind ocelli
Length of postocular setae: not alternating short and long setae
Number of ocellar setae: 3
Prothorax
Number of pairs of anteromarginal minor setae: 2-3
Number of pairs of long anteroangular setae: 1-2
Number of pairs of long posteroangular setae: 2
Number of pairs of elongate pronotal setae: 4-5
Number of pairs of posteromarginal minor setae: 4-5
Pronotal blotch or internal apodeme: absent
Pronotum shape: broadly rectangular
Pronotum posteromarginal/posteroangular setae: S2 longer than S3, not equal in length
Mesothorax
Mesosternal furca: with median spinula
Metathorax
Metanotal campaniform sensilla: present
Metanotal median setae: S1 at anterior margin
Metanotum with dominant sculptured triangle medially: absent
Metasternal furca: without spinula
Shape of metathoracic furca: transverse, V-shaped
Metanotal median setae length: longer than lateral metanotal setae
Sculpture of metanotum median area: transverse at anterior, but equiangular reticulations, or irregular longitudinal, or equiangular reticulations
on posterior half
Wings
Fore and hind wings: present, more than half as long as abdomen (macropterous)
Fringe cilia arising: from sockets
Fore wing veins: present
Fore- and hind wing surface: covered with microtrichia
Apex of fore wing: with prominent terminal setae
Fore wing anterior margin (costal vein): with setae and cilia but cilia longer than setae
Fore wing costal fringe cilia: arising at anterior margin of wing
Fore wing first vein: distinct from costal vein
Fore wing first vein setal row: complete, with setae closely and uniformly spaced
Fore wing second vein setal row: complete, setae uniformly spaced
Fore wing shape: mainly parallel sided or margins run continuously towards each other
Fore wing surface: not reticulate
Fringe cilia on posterior margin near apex: distinctly wavy (undulated)
Length of fore wing costal setae at middle of wing: longer than half of median wing width
Shape of fore wing apex: with mainly posterior margin curved to join anterior margin
Fore wing extreme apex color: pale
Fore wings: uniformly pale or weakly shaded
Legs
Fore tibia: not prolonged around fore tarsus
Mid and hind tarsi: with two segments
Color of fore tarsi: brown or pale or yellow, sometimes apical shaded or brown
Abdomen
Pleurotergites: not covered in microtrichia
Sternite II: with marginal setae but no discal setae
Sternites IV, V and VI: with marginal setae but no discal setae
Sternite VII: with marginal setae but no discal setae
Surface of lateral thirds of abdominal tergites: without regular rows of fine microtrichia
Tergites II to VII median setal pair: no more than 0.3 as long as median length of tergite
Craspedum on tergites IV to VI: absent
Tergites IV and V median setal pair: shorter than distance between their bases
Tergites V to VII: with ctenidia laterally
Craspedum on tergite VIII: without craspedum medially and toothlike microtrichia laterally
Tergite VIII ctenidia: anterolateral to spiracle
Tergite VIII posteromarginal comb of microtrichia: present and complete medially
Tergite VIII shape of posteromarginal microtrichia: long or short, slender and regular on broadly triangular bases
Tergite X: not tubular, longitudinally incomplete
Setae on abdominal tergite X: all setae slender

Similar or related species
Frankliniella occidentalis is very similar to Frankliniella borinquen with differences in the shape of pedicel of antennal segment III (Frankliniella borinquen has a pedicel swollen, with edged ring surmounted by a distinctive swelling and a slightly flared collar; Frankliniella occidentalis as well as Frankliniella schultzei and Frankliniella williamsi with a simple pedicel), and length of terminal antennal segment (Frankliniella borinquen with antennal segment VIII equal in length to or shorter than segment VII; other three species with antennal segment VIII longer than segment VII). In Frankliniella occidentalis, ocellar setae III on head are about as long as distance between external margins of hind ocelli and in Frankliniella borinquen as long as distance between midpoints of hind ocelli, whereas in Frankliniella schultzei and Frankliniella williamsi they are about as long as distance between hind ocelli. In Frankliniella borinquen as well as Frankliniella schultzei the length of postocular setae IV are about as long as distance between hind ocelli (in Frankliniella occidentalis postocular setae IV are longer and in Frankliniella williamsi distinctly shorter than distance between hind ocelli).
In Frankliniella occidentalis, Frankliniella borinquen and Frankliniella williamsi ocellar setae III arising on anterior margins of or just within anterior margins of ocellar triangle, and campaniform sensilla on metanotum normally present (only Frankliniella schultzei with ocellar setae III arising very close together between anterior margins of hind ocelli, and without metanotal campaniform sensilla). As in Frankliniella borinquen, females of Frankliniella occidentalis with a complete posteromarginal comb of short microtrichia arising from triangular bases on tergite VIII (but in Frankliniella occidentalis microtrichia short or long). In constrast, Frankliniella williamsi tergite VIII with posteromarginal comb of long and fine, closely spaced microtrichia on broadly triangular bases, and Frankliniella schultzei comb weakly developed or absent. Compared to Frankliniella williamsi which has 1 or 2 median discal setae in addition to marginal setae on sternite II, other species of Frankliniella possess sternite II without median discal setae.
Species of Frankliniella are similar to species of Thrips, Stenchaetothrips, Microcephalothrips abdominalis, Larothrips dentipes and Fulmekiola serrata, in having tergites V-VIII with pairs of ctenidia laterally, but in these species ctenidia of the tergite VIII arranged posteromedial to the spiracle, whereas species of Frankliniella have ctenidia on tergite VIII anterolateral to the spiracle.
Biology
Life history
Dependent on temperature, life cycle from egg to adult can range from 15 (30°C) to 44 (15°C) days, and adults can live for 28 to 71 days. The number of eggs that an adult thrips can lay range from 24 to 96 and there can be 12-15 generations in constant warm temperatures such as in greenhouses (OEPP/EPPO 1989). Different diets have effects on the oviposition rate (Kumm & Moritz 2010; Steinbach et al. 2012). Male killing bacteria, like Wolbachia were not detected in Wester Flower Thrips (WFT) (Kumm & Moritz 2008).
Host plants
Polyphagous, feeds on all plant parts i.e., flowers, fruit, seeds, stems and leaves of numerous plant material such as vegetables, fruits, potted flowering plants and nursery stock.
Crops: African nightshade, amaranth, babycorn, beans (broad bean, common bean, French bean, hyacinth bean, Lima bean), beet root, broccoli, cabbage, capsicum, cassava, chillies, courgettes, cucurbit, dolichos, eggplant,export flowers (Ornithogalum arabicum, Eryngium sp., Lupinus sp., Gomphocarpus semilunatus (mobydic)), kale, leek, maize, onion, papaya, peas, potato, pumpkin, squash, red gram, thorn apple, tomato, wild sunflower, wheat.
Weeds: Achyranthes aspera, Ajuga remota, Bidens pilosa, Chenopodium sp., Conyza bonariensis, Crotolaria sp., Datura suaveolens, Erlangea calycina, Galinsoga parviflora, Nycandra physalodes, Senna didymobotrya, Sonchus oleraceus, Tagetes minuta, Tithonia diversifolia.
Feeding and oviposition preference experiments with Frankliniella occidentalis indicated that Cucurbita pepo and G. parviflora may serve as potential sources of WFT outbreaks within French bean fields of Kenya (Nyasani et al. 2012b).
Vector capacity
Tomato spotted wilt virus (TSWV)
In East Africa Frankliniella occidentalis vector Tomato Spotted Wilt Virus on tomato and cucurbits (Wangai et al. 2001; Ramkat et al. 2006; Ramkat et al. 2008).
Impatiens necrotic spot virus (INSV)
Groundnut ringspot virus (GRSV)
Tomato chlorotic spot virus (TCSV)
Chrysanthemum stem necrosis virus (CSNV)
Also known to transmit Fusarium moniliforme (a fungus that causes ear rot of corn) and Erwinia amylovora (fire blight bacteria).
Damage and symptoms
Deformation of young seedlings and fruit and discoloration, silvering and bleaching of leaves, flowers and fruit. Fruits scarred very early in development causing disfigurement as fruit expands.
Detection and control strategies
Frankliniella occidentalis are attracted to both yellow and blue sticky traps, which could be used for effective monitoring of their dynamics in the field. They are also attracted to semiochemicals such as the aggregation pheromone (Hamilton et al 2005) and the flower based kairomones (Teulon et al 2007; Muvea, 2011).
Intercropping French bean with baby corn/ Irish Potato/ Sunflower at ratio of 1: 4 reduced the incidence of thrips and enhanced the marketable yield by nearly 50% (Nyasani et al. 2012a).
The biological agent Amblyseius swirkii (Acari: Phytoseiidae) have been used to control Frankliniella occidentalis on sweet pepper in Turkey (Kutuk et al. 2011). Other natural enemies used for their management include Neoseiulus cucumeris (=Amblyseius cucumeris), Amblyseius degenerans, Hypoaspis miles and Hypoaspis aculeifer (Acari: Laelapidae), the minute pirate bug Orius insidiosus (Hemiptera: Anthocoridae), the entomopathogenic or insect-killing nematode Steinernema feltiae (Nematoda: Steinernematidae), and the entomopathogenic fungus Beauveria bassina (Cloyd 2009). Metarhizium. anisopliae isolate ICIPE 69 was found to be effective against both the larvae and adults of Frankliniella occidentalis (Niassy et al. 2012b). This fungus is compatible with imidacloprid or thiamethoxam (Niassy et al. 2012c). Metarhizium anisopliae isolate ICIPE 69 developed for thrips management, is fully registered and already commercialized in Ghana under the name of Campaign®, and is being registered in several other African countries (Mozambique, Ethiopia, Kenya and South Africa) (Ekesi et al. 2011). A semiochemical-baited autoinoculation device treated with Metarhizium anisopliae for control of Frankliniella occidentalis on French bean is currently under development (Niassy et al. 2012a).
Additional notes
Beneficial aspects: Feeds on mite eggs.
Biogeography
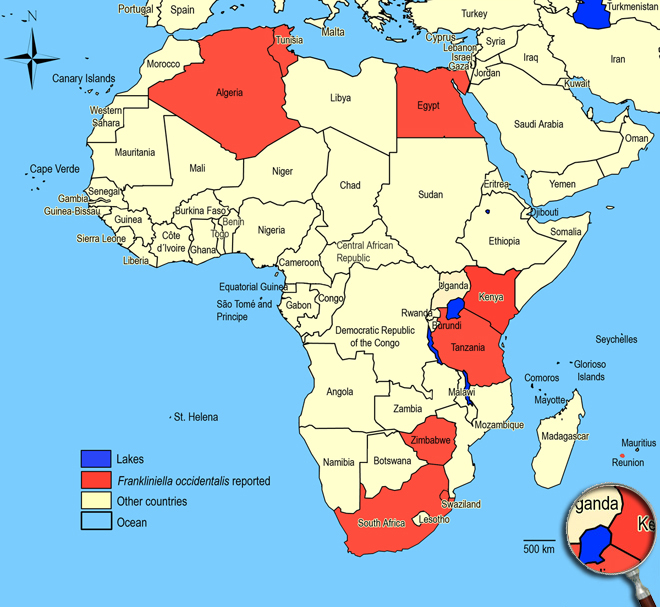
Originally from the South Western States of the USA, the western flower thrips is now widespread around the world. It has become established in areas with a Mediterranean climate, but in colder areas it is found in greenhouses. It is not a pest in the lowlands of the wet tropics, although it is sometimes abundant at high elevations in such countries, including Kenya and Malaya. In Africa the pest is reported from Kenya, Tanzania, Egypt, Algeria, Tunisia, South Africa (Gauteng: Krugersdorp; Limpopo: Letsitele; Western Cape) Zimbabwe, Uganda and Reunion islands.
African countries where Frankliniella occidentalis has been reported
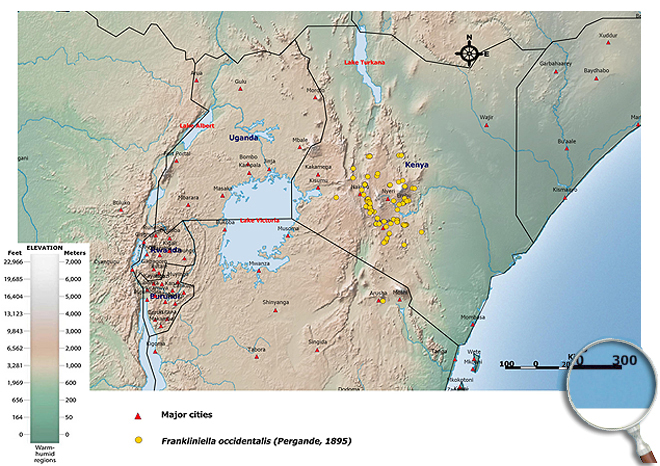
Occurence of Frankliniella occidentalis in East Africa
Please click here for survey sites of all observed thrips species of Kenya, Tanzania and Uganda.
Click here for locations of Frankliniella occidentalis in parts of East Africa.

Bibliography
Boissot N, Reynaud B & Letourmy P (1998). Temporal analysis of western flower thrips (Thysanoptera: Thripidae) population dynamics on Reunion Island. Environmental Entomology. 27 (6): 1437-1443
Cavalleri A & Mound LA (2012). Toward the identification of Frankliniella species in Brazil (Thysanoptera, Thripidae). Zootaxa 3270: 1-30
Childers CC & Beshear RJ (1992). Thrips (Thysanoptera) species associated with developing citrus flowers in Florida and a key to adult terebrantian females. Journal of Entomological Science. 27 (4): 392-412
Cloyd RA (2009). Western flower thrips (Frankliniella occidentalis) management on ornamental crops grown in greenhouses: Have we reached an impasse? Pest Technology. 3 (1): 1-9
Daiber KC (1992). Insects and nematodes that attack cole crops in Southern Africa - a research review. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz. 99 (4): 430-440
de Villiers M & Pringle KL (2007). Seasonal occurrence of vine pests in commercially treated vineyards in the Hex River Valley in the Western Cape Province, South Africa. African Entomology. 15 (2): 241-260
Ekesi S, Chabi-Olaye A, Subramanian S & Borgemeister C (2011). Horticultural Pest Management and the African economy: Successes, Challenges and Opportunities in a Changing global environment. Acta Hort. (ISHS) 911:165-183
Gaum WG, Giliomee JH & Pringle KL (1994). Life history and life tables of western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae), on English cucumbers. Bulletin of Entomological Research. 84 (2): 219-224
Gaum WG, Giliomee JH & Pringle KL (1994). Resistance of some rose cultivars to the western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae). Bulletin of Entomological Research. 84 (4): 487-492
Giliomee JH (1989). First record of western flower thrips, Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae) from South Africa. Journal of the Entomological Society of Southern Africa. 52 (1): 179-180
Gitonga LM, Lohr B, Overholt WA, Magambo JK & Mueke JM (2002). Temperature-dependent development of Megalurothrips sjostedti and Frankliniella occidentalis (Thysanoptera: Thripidae). African Entomology. 10 (2): 325-331
Grové T, Giliomee JH & Pringle KL (2001). Thrips (Thysanoptera) species associated with mango trees in South Africa. African Entomology. 9 (2): 153-162
Hood JD (1914). On the proper generic names of certain Thysanoptera of economic importance. Proceedings of the Entomological Society of Washington. 16: 34-44
Karnkowski W & Trdan S (2002). Diagnostic protocols for regulated pests - Protocoles de diagnostic pour les organismes réglementés Frankliniella occidentalis. Bulletin OEPP/EPPO Bulletin. 32 (2): 281-292
Kasina J, Nderitu J, Nyamasyo G, Olubayo F, Waturu C, Obudho E & Yobera D (2006). Diurnal population trends of Megalurothrips sjostedti and Frankliniella occidentalis (Thysanoptera: Thripidae) and their natural enemies on French bean Phaseolus vulgaris (Fabaceae). International Journal of Tropical Insect Science. 26 (1): 2-7
Kasina J, Nderitu J, Nyamasyo G, Olubayo F, Waturu C, Obudho E & Yobera D (2006). Evaluation of companion crops for thrips (Thysanoptera: Thripidae) management on French bean Phaseolus vulgaris (Fabaceae). International Journal of Tropical Insect Science. 26 (2): 121-125
Kirk WDJ & Terry LI (2003). The spread of the western flower thrips Frankliniella occidentalis (Pergande). Agricultural and Forest Entomology. 5 (4): 301-310
Kumm S & Moritz G (2008). First detection of Wolbachia in arrhenotokous populations of thrips species (Thysanoptera: Thripidae and Phlaeothripidae) and its role in reproduction. Environ. Entomol. 37(6): 1422-1428
Kumm S & Moritz G (2010). Life cycle variation, inlcuding female production by virgin females in Frankliniella occidentalis (Thysanoptera: Thripidae). Journal of Applied Entomology. 134 (6): 491-497
Kutuk H, Yigit A, Canhilal R & Karacaoglu M (2011). Control of western flower thrips (Frankliniella occidentalis) with Amblyseius swirski on greenhouse pepper in heated and unheated plastic tunnels in the Mediterranean region of Turkey. African Journal of Agricultural Research. 6 (24): 5428-5433
Lewis T (1973). Thrips: their biology, ecology and economic importance. Academic Press Inc., London Ltd., 349 pp
Lewis T (1997). Thrips as crop pests. CAB International, Wallingford, 740 pp
Maniania NK, Ekesi S, Löhr B & Mwangi F (2003). Prospects for biological control of the western flower thrips, Frankliniella occidentalis, with the entomopathogenic fungus, Metarhizium anisopliae, on chrysanthemum. Mycopathologia. 155 (4): 229-235
Milne JR, Milne M & Walter GH (1997). A key to larval thrips (Thysanoptera) from granite belt stonefruit trees and a first description of Pseudanaphothrips achaetus (Bagnall) larvae. Australian Journal of Entomology. 36 (4): 319-326
Morgan AC (1925). Six new species of Frankliniella and a key to the American species. Canadian Entomologist. 57 (6): 136-147
Moritz G (2006). Thripse. Pflanzensaftsaugende Insekten, Bd. 1, (1. Auflage). Westarp, Hohenwarsleben, 384 pp. ISBN-13: 978 3 89432 891 7
Moritz G, Morris DC & Mound LA (2001). ThripsID - Pest thrips of the world. ACIAR and CSIRO Publishing Collingwood, Victoria, Australia, CDROM ISBN 1 86320 296 X
Moritz G, Mound LA, Morris DC & Goldarazena A (2004). Pest thrips of the world - an identification and information system using molecular and microscopical methods. Centre for Biological Information Technology, University of Queensland, Australia, CDROM ISBN 1 86499 781 8
Moritz G, O'Donnell C & Parrella M (2009). Pest thrips of North America. Centre for Biological Information Technology, University of Queensland, Australia, CDROM ISBN-13: 978 1 86499 940 2
Moulton D (1911). Synopsis, catalogue and bibliography of North American Thysanoptera, with descriptions of new species. Technical Series, USDA, Bureau of Entomology. 21: 1-56
Moulton D (1935). New California Thysanoptera. Pan-Pacific Entomologist. 11: 170-174
Moulton D (1948). The genus Frankliniella Karny, with keys for the determination of species (Thysanoptera). Revista de Entomologia, Rio de Janeiro. 19 (1-2): 55-114
Mound LA & Kibby G (1998). Thysanoptera: An identification guide, (2nd edition). CAB International, Wallingford and New York, 70 pp
Mound LA & Marullo R (1996). The thrips of Central and South America: An introduction (Insecta: Thysanoptera). Memoirs on Entomology, International, Vol. 6. Associated Publishers, Gainsville, 487 pp
Muvea AM (2011). The potential of coloured sticky traps with kairomonal attractants (LUREM-TR) in management of thrips on Tomato and French beans. Unpublished thesis, Jomo Kenyatta University of Agriculture and Technology, Nairobi, Kenya, 107 pp
Nakahara S (1991). Systematics of Thysanoptera, pear thrips and other economic species, pp. 41-59. In Parker BL, Skinner M & Lewis T [eds.], Towards understanding Thysanoptera. USDA, General Technical Report NE-147
Nakahara S (1997). Annotated list of the Frankliniella species of the world (Thysanoptera: Thripidae). Contributions on Entomology, International. 2 (4): 355-389
Nderitu JH, Kasina MJ, Nyamasyo GN, Waturu CN & Aura J (2008). Management of thrips (Thysanoptera: Thripidae) on French beans (Fabaceae) in Kenya: Economics of insecticide applications. Journal of Entomology. 5 (3): 148-155
Niassy S, Maniania NK, Subramanian S, Gitonga LM & Ekesi S (2012a). Performance of a semiochemical-baited autoinoculation device treated with Metarhizium anisopliae for control of Frankliniella occidentalis on French bean in field cages. Entomologia Experimentalis et Applicata 142: 97-103.
Niassy S, Maniania NK, Subramanian S, Gitonga LM, Mburu DM, Masiga D & Ekesi S (2012b). Selection of promising fungal biological control agent of the western flower thrips Frankliniella occidentalis (Pergande) Letters in Applied Microbiology 54: 487-493.
Niassy S, Maniania NK, Subramanian S, Gitonga LM, Maranga R, Obonyo AB & Ekesi S (2012c) Compatibility of Metarhizium anisopliae isolate ICIPE 69 with agrochemicals used in French bean production. International Journal of Pest Management 58, 131-137.
Nickle DA (2003). A checklist of commonly intercepted thrips (Thysanoptera) from Europe, the Mediterranean, and Africa at U.S. ports-of-entry (1983-1999). Part I. Key to genera. Proceedings of the Entomological Society of Washington. 105 (1): 80-99
Nickle DA (2004). Commonly intercepted thrips (Thysanoptera) from Europe, the Mediterranean, and Africa at U.S. ports-of-entry. Part II. Frankliniella Karny and Iridothrips Priesner (Thripidae). Proceedings of the Entomological Society of Washington. 106 (2): 438-452
Nyasani JO, Meyhöfer R, Subramanian S & Poehling H.-M (2012a). Effect of intercrops on thrips species composition and population abundance on French beans in Kenya. Entomologia Experimentalis et Applicata 142: 236-246
Nyasani JO, Meyhöfer R, Subramanian S & Poehling H.-M. (2012b). Feeding and oviposition preference of Frankliniella occidentalis for crops and weeds in Kenyan French bean fields. Journal of Applied Entomology DOI: 10.1111/j.1439-0418.2012.01723.x
OEPP/EPPO (1989). Data sheets on quarantine organisms No. 177, Frankliniella occidentalis. Bulletin OEPP/EPPO Bulletin. 19: 725-731
Palmer JM, Mound LA & Du Heaume GJ (1989). 2. Thysanoptera, 73 pp. In Betts CR [ed.], CIE Guides to insects of importance to man. CAB International, Wallingford, Oxon, UK
Pergande T (1895). Observations on certain Thripidae. Insect Life. 7 (5): 390-395
Priesner H (1925). Neue Thysanopteren. Deutsche Entomologische Zeitschrift. 1925: 13-28
Priesner H (1932). Neue Thysanopteren aus Mexiko, gesammelt von Prof. Dr. A. Dampf. Teil 1. Wiener Entomologische Zeitung. 49: 170-185
Ramkat RC, Wangai AW, Rapando PN & Lelgut DK (2008). Cropping system influences Tomato spotted wilt virus disease development, thrips population and yield of tomato (Lycoperscion escullentum). Journal of Applied Biology, 153,373-380
Ramkat RC, Wangai AW, Ouma JP, Rapando, PN & Lelgut DK (2006). Effect of mechanical inoculation of tomato spotted wilt tospovirus disease on disease severity and yield of greenhouse raised tomatoes. Asian Journal of Plant Sciences, 5,607 - 612
Stannard LJ (1968). The thrips, or Thysanoptera, of Illinois. Illinois Natural History Survey Bulletin. 29 (4): 214-552
Steinbach D, Kumm S & Moritz G 2012. Effects of different diets on oviposition rate of Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae). Acta Phytopathologica et Entomologica Hungarica 47: 151-159
Teulon DAJ, Butler RC, James DE & Davidson MM 2007. Odour-baited traps influence thrips capture in proximal unbaited traps in the field. Entomologia Experimentalis et Applicata, 123: 253-262
Treherne RC (1924). Thysanoptera known to occur in Canada. Canadian Entomologist. 56: 82-88
Vayssières JF, Delvare G, Maldès JM & Aberlenc HP (2001). Inventaire preliminaire des arthropodes ravageurs et auxiliaires des cultures maraicheres sur ľIle de la Réunion. Insect Science and its Application. 21 (1): 1-22
Wangi AW, Mandal B, Pappu HR & Kilonzo S (2001). Outbreak of tomatoes spotted wilt virus in tomato in Kenya. Plant Diseases, 85, 1123
Waterhouse DF & Norris KR (1889). Biological control - Pacific prospects. Supplement 1. Australian Centre for International Agricultural Research, Inkata Press, Melbourne, 123 pp
Zhang Z-J , Wu Q-J, Li X-F, Zhang Y-J, Xu B-Y & Zhu G-R (2007). Life history of western flower thrips, Frankliniella occidentalis (Thysan., Thripae), on five different vegetable leaves. Journal of Applied Entomology. 131 (5): 347-354
zur Strassen R (2003). Die terebranten Thysanopteren Europas und des Mittelmeer-Gebietes. Die Tierwelt Deutschlands und der angrenzenden Meeresteile nach ihren Merkmalen und nach ihrer Lebensweise, 74. Teil. Goecke & Evers, Keltern, Germany, 277 pp
zur Strassen R (2006). Checklist of the Thysanoptera (Insecta) of southern Africa. African Entomology. 14 (1): 63-68
----
Web links
Mound´s Thysanoptera pages
Thysanoptera Checklist
ICIPE Thrips survey sites
UNI Halle & Thrips sites
Thrips of California