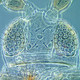
Scirtothrips aurantii Faure, 1929
Thripinae, Thripidae, Terebrantia, Thysanoptera
Figures
Fig. 1: 8-segmented antenna, segments III and IV with forked sense cone, terminal segments VI-VIII
Fig. 2: Head dorsal with ocellar triangle
Fig. 3: Head and pronotum
Fig. 4: Pronotum
Fig. 5: Meso- and metanotum
Fig. 6: Fore wing and fore wing distal region
Fig. 7: Hind femur of male
Fig. 8: Sternites III and IV
Fig. 9: Tergites VII and VIII
Fig. 10: Tergites VIII-XI
Introduction and recognition
Scirtothrips aurantii is a polyphagous, leaf and fruit feeding thrips and a serious economic pest, especially of citrus and mangoes. Both sexes fully winged. Body mainly yellow, with dark brown antecostal ridge on tergites and sternites, and small brown tergal area medially; antennal segment I white, III & IV grey, II and V-VIII brown; major setae dark; fore wings light brown or pale, but clavus shaded. Antennae 8-segmented; segments III & IV with constricted apical neck and sense cone forked and stout (Fig. 1). Head wider than long; ocellar triangle and postocular region with closely spaced sculpture lines; 3 pairs of ocellar setae, pair I short and behind base of scape and in front of anterior ocellus, pair III arising close together between anterior margins of hind ocelli (Fig. 2 and 3). Pronotum with closely spaced transverse sculpture lines; posterior margin with 4 pairs of setae, S2 prominent and elongate, S3 moderately long (Fig. 3 and 4). Meso- and metafurca with spinula. Metanotal median area with transverse sculpture at anterior, and posterior half with irregular longitudinal reticulations; median setae arising at anterior margin; campaniform sensilla absent (Fig. 5). Mid and hind tarsi 2-segmented. Fore wing first vein with 3 setae on distal half; second vein with 2-5 widely spaced setae; posteromarginal cilia wavy; clavus with 4 veinal setae (Fig. 6). Tergites III-VII with median setae small but close together; II-VIII with lateral thirds covered in closely spaced, regular rows of fine microtrichia, these microtrichial fields with 3 discal setae, posterior margin of these tergites with fine comb laterally ; VIII with comb complete across posterior margin, and a patch of microtrichia anteromedially, median setae long(Fig. 9); IX with no discal microtrichia (Fig. 10). Sternites without discal setae; completely covered with rows of microtrichia extending fully across discal area; posterior margins with comb of short microtrichia between marginal setae; median setae on VII arising at posterior margin (Fig. 8).
Male similar to female but smaller; tergite IX posterior angles bearing pair of stout curved processes (drepanae) extending across tergite X; hind femora with comb-like row of stout setae (Fig. 7); sternites without glandular areas.
Second instar larva white, antennal segment II grey; tergites covered in irregularly arranged dot-like sculpture, pronotum with distinctive reticulate markings; setae capitate on head, posterior angles of pronotum, and tergite X, remaining setae small and acute; abdominal spiracles small.
Taxonomic identity
Species
Scirtothrips aurantii Faure, 1929
Taxonomic history
Scirtothrips acaciae Moulton, 1930
Common name
South African citrus thrips
Citrus thrips
Present taxonomic position
Family: Thripidae Stephens, 1829
Subfamily: Thripinae (Stephens) Karny, 1921
Genus: Scirtothrips Shull, 1909
Genus description
The genus Scirtothrips Shull, 1909
Scirtothrips currently includes more than 100 described species from temperate, subtropical and tropical areas of the world. Several species are serious crop pests. All of them having many regular rows of microtrichia on the lateral sides of the tergites (species of the genus look a like minute fur-bearing animals), and a complete posteromarginal comb of microtrichia on tergite VIII. These are small, usually pale yellow thrips with 8-segmented antennae, segments III and IV with forked sense cone, ovipositor well sklerotized and makes slide praperation often very complicate. Surface of pronotum closely transversely striate, the fore wings are narrow with only a few distal setae on the first vein and a few apical setae on the second vein. Mound & Palmer (1981) present a key to the major pest species, and Mound & Marullo (1996) a key to the species of Central America. Johansen & Mojica-Guzmán (1998) described 33 species from Mexico, particularly from mango and avocado trees, but doubts have been expressed concerning the systematical validity of some of these species (Mound & zur Strassen 2001). Hoddle & Mound (2003) provided information on 21 Scirtothrips species from Australia, and Rugman-Jones et al. (2006) produced a molecular key to several pest species in this genus. Relationships of various Scirtothrips species based on molecular data are further considered by Hoddle et al. (2008) and a molecular identification method was given by Moritz et al. (2000) and used for the first time in combintion with LucID (Moritz et al. 2004).
Species description
Typical key character states of
Scirtothrips aurantii
Coloration and body sculpture
Body color: mainly pale to yellow, or with some darker markings
Surface of head, pronotum and fore legs: without obvious or with weakly reticulate sculpture
Antennae
Number of antennal segments: 8
Antennal segment I: without any setae on dorsal apical margin
Antennal segment II: without an exceptionally long seta at the inner apex
Antennal segment II shape: symmetric
Antennal segment III shape: symmetric
Length of antennal segment III and IV: antennal segment III similar in length to segment IV
Form of sense cones on antennal segments III and IV: emergent and forked on segments III and IV
Antennal segment IV and V: without a hyaline ring near the base
Forked sense cone on antennal segment IV: scarcely extending beyond base of segment V
Antennal segment VI bears: not a remarkably dagger-shaped sensorium
Head
Distance between bases of ocellar setae III: same or less than width of first ocellus
Head: not prolonged in front of compound eyes
Ocellar setae I: present
Length of ocellar setae I: shorter than setae III
Ocellar setae III: arising on anterior margin of, or between hind ocelli
Ocelli: present
Length of postocular setae: not alternating short and long setae
Ocellar setae I position: short and behind base of scape and in front of anterior ocellus
Number of ocellar setae: 3
Prothorax
Number of pairs of long posteroangular setae: 1
Number of pairs of elongate pronotal setae: 1
Pronotal blotch or internal apodeme: absent
Pronotum shape: broadly rectangular
Pronotum surface: with transverse striate sculpture
Pronotum posteromarginal/posteroangular setae: S2 longer than S3, not equal in length
Mesothorax
Mesosternal furca: with median spinula
Metathorax
Metanotal campaniform sensilla: absent
Metanotum with dominant sculptured triangle medially: absent
Metasternal furca: with spinula
Metanotal median setae: S1 at anterior margin
Shape of metathoracic furca: transverse,V-shaped
Wings
Fore and hind wings: present, more than half as long as abdomen (macropterous)
Fringe cilia arising: from sockets
Fore wing veins: present
Fore- and hind wing surface: covered with microtrichia
Apex of fore wing: with prominent terminal setae
Fore wing anterior margin (costal vein): with setae and cilia but cilia longer than setae
Fore wing costal fringe cilia: arising at anterior margin of wing
Fore wing first vein: distinct from costal vein
Fore wing first vein setal row: incomplete, with setae not closely and uniformly spaced
Fore wing second vein setal row: incomplete, with setae not closely and uniformly spaced
Fore wing shape: mainly parallel sided or margins run continuously towards each other
Fore wing surface: not reticulate
Fringe cilia on posterior margin near apex: distinctly wavy (undulated)
Length of fore wing costal setae at middle of wing: longer than half of median wing width
Shape of fore wing apex: with mainly posterior margin curved to join anterior margin
Fore wings: uniformly light brown or uniformly pale or weakly shaded
Legs
Fore tibia: not prolonged around fore tarsus
Mid and hind tarsi: with two segments
Color of fore tarsi: pale or yellow, sometimes apical shaded or brown
Abdomen
Pleurotergites: with many rows of fine microtrichia
Sternite II: with marginal setae but no discal setae
Sternites IV, V and VI: with marginal setae but no discal setae
Sternites V and VI microtrichia: extending fully across discal area
Sternite VII median posteromarginal setae S1: arising at posterior margin
Sternite VII: with marginal setae but no discal setae
Surface of lateral thirds of abdominal tergites: with many regular rows of fine microtrichia
Tergites II to VII median setal pair: no more than 0.3 as long as median length of tergite
Craspedum on tergites IV to VI: absent
Markings on tergites IV to VI: with shaded areas medially
Tergites V to VII: without ctenidia laterally, but sometimes with rows of microtrichia
Craspedum on tergite VIII: without craspedum medially and toothlike microtrichia laterally
Tergite VIII ctenidia: without paired ctenidia laterally, sometimes with irregular microtrichia
Tergite VIII posteromarginal comb of microtrichia: present and complete medially
Tergite VIII microtrichia anteromedially: present
Tergite IX microtrichia medially: absent
Tergite X: not tubular, longitudinally incomplete
Setae on abdominal tergite X: all setae slender

Similar or related species
Compared to any other member of the genus, the male of Scirtothrips aurantii has a comb of stout setae on the inner margin of the hind femur. Most of the species have a dark brown antecostal ridge on tergites and sternites, a small brown tergal area medially, and 3 setae on tergal microtrichial field laterally (except for Scirtothrips mangiferae which has no dark brown antecostal ridge on tergites and sternites nor any distinctive color patterns on tergites, and 4-6 setae on tergal microtrichial field laterally), the metanotal median setae arising at anterior margin (only in Scirtothrips dorsalis metanotal median setae arising behind anterior margin), and the species usually have a mainly yellow body color, fore wings uniformly light brown or pale, and a patch of microtrichia anteromedially on tergite VIII (except for Scirtothrips kenyensis with a light brown body color, uniformly dark brown fore wings, and without microtrichia anteromedially on tergite VIII). Scirtothrips aurantii differs from other species in having ocellar setae III arising between anterior margin of hind ocelli (Scirtothrips dorsalis with pair III conspicuously between median points of hind ocelli; Scirtothrips kenyensis and Scirtothrips mangiferae with ocellar setae pair III arising anterior to tangent of anterior margin of hind ocelli). The fore wing posterior margin cilia of Scirtothrips aurantii and Scirtothrips mangiferae are at least in distal part undulated (Scirtothrips dorsalis and Scirtothrips kenyensis with straight cilia). Scirtothrips aurantii as well as Scirtothrips kenyensis have no microtrichia medially on tergite IX (in Scirtothrips dorsalis and Scirtothrips mangiferae present). The sternites of Scirtothrips aurantii and Scirtothrips dorsalis exhibits a strong microtrichial field extending fully across discal area (compared to Scirtothrips kenyensis and Scirtothrips mangiferae with microtrichia restricted to lateral thirds of discal area).
Kenyattathrips katarinae is related to species of Scirtothrips. All of them have many regular, closely spaced rows of fine microtrichia on the lateral sides of the tergites and a complete posteromarginal comb on tergite VIII, antennal segments III & IV with forked sense cone, and the pronotum on these thrips is closely and transversely striate. Compared to Kenyattathrips katarinae, all species of Scirtothrips have 8-segmented antennae, antennal segment II without an exeptionally long seta at the inner apex, ocellar setal pair I short and behind base of scape and in front of anterior ocellus, only 1-2 pairs of elongate pronotal setae and no long anteromarginal setae on the pronotum, metanotum reticulated medially, fore wings second vein with a few apical setae, and tergite VII bears the posteromarginal comb of microtrichia only laterally. Whereas Kenyattathrips katarinae has 7-segmented antennae, antennal segment II with an exceptionally long seta at the inner apex, a long ocellar setal pair I placed far forward on the inter-antennal projection, 3-4 pairs of elongate pronotal setae (1 pair anteromarginally, 1 pair moderately elongate laterally, 2 pairs posteromarginally), a metanotum without or with weakly sculpture medially, an almost complete row of setae on fore wing second vein, and a complete posteromarginal comb of microtrichia on tergite VII. Furthermore, species of Scirtothrips are similar to Neohydatothrips samayunkur and Hydatothrips adolfifriderici in having the surface of lateral thirds of tergites bearing many regular rows of fine microtrichia. But Neohydatothrips samayunkur and Hydatothrips adolfifriderici have ocellar setae III on head arising on anterior margin, or in front of, ocellar triangle, fore wings with a complete row of setae on the first vein and without or only 2 setae on the second vein, and a distinctive colored and/or sculptured area on the pronotum, the pronotal blotch. Like species of the genus Scirtothrips, also Florithrips has pleurotergites and tergites laterally covered with fine microtrichia, but in Scirtothrips these are arranged in closely spaced rows, whereas in Florithrips traegardhi these microtrichia extend along lines of sculpture. In addition, tergites II-VII of Scirtothrips species have a fine comb laterally at posterior margin, and sternites covered with rows of microtrichia, which lacking in Florithrips traegardhi.
Biology
Life history
Egg, larval and pupal stages last 1-2 days, 1-2 weeks and 1-1.5 weeks respectively (Gahukar 2004). Life history can be completed in less than 30 days.
Host plants
Highly polyphagous; recorded from plants in about 30 different families.
Crops: African nightshade, amaranth, asparagus, banana, capsicum, cashew, cassava, castor, chillies, citrus, cotton, French beans, kale, mango, orange, red gram, spinach, sweet potato, tea.
Weeds: Bidens pilosa, Datura suaveolens, Galinsoga parviflora, Leonotis nepetifolia, Nycandra physalodes, Tagetes minuta.
Vector capacity
None identified, but possible mechanical distribution of phytopathogenic fungi and bacteria.
Damage and symptoms
All of the members of this group feed on the leaves of their plant hosts and are quite cryptic in habit. Scirtothrips aurantii breeding on young tissues of leaves and fruits. Feeding results in distortion of young leaves, but of greater economic importance is the surface scarring on citrus fruits leading to downgrading of a crop. Feeding by larvae and adult thrips on fruit results in a ring of brownish scales around the base of the fruit (Gahukar 2004).
Detection and control strategies
Yellow non- fluorescent traps were more effective than flourescent traps for monitoring Scirtothrips aurantii (Grout & Richards 1990). Single application of synthetic pesticides such as Fipronil, abamectic and tartar emetic were effective against Scirtothrips aurantii on mango, however resulted in secondary pest outbreaks. Biorationals such as Neem oil, Lime sulfur and Natural Pyrethrum were effective with frequent applications and did not result in secondary pest outbreak (Le Lagadec & Bruwer 2002).
Two phytoseiid mites, Euseius addoensis addoensis (Grout & Stephen 1994), and E. citri (Grout 1994) are major indigenous predators of citrus thrips in South Africa.
The eulophid parasitoid, Goetheana incerta naturally parasitizes Scirtothrips aurantii (Triapitsyn, 2005).
Additional notes
-
Biogeography
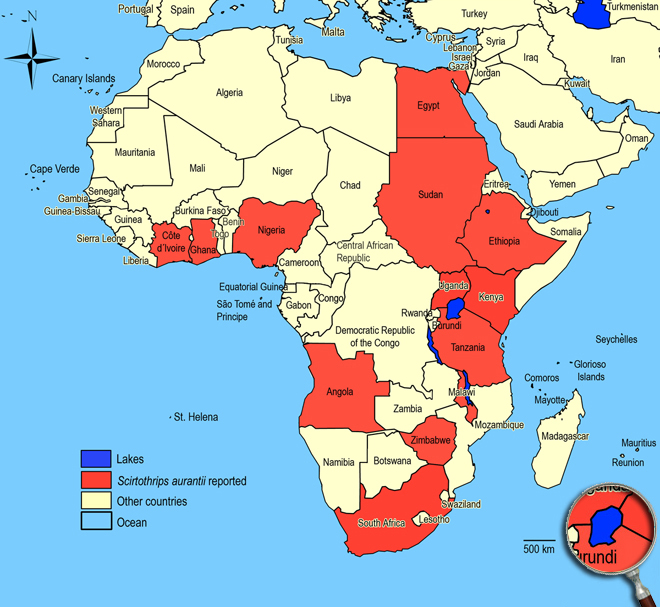
Subtropics and tropics of Africa, introduced to Queensland (Australia). Angola, Egypt, Ethiopia, Ghana (Yegi), Ivory Coast, Kenya (Mbita), Malawi, Nigeria (Ibadan), South Africa (Mpumalanga: Malelane; KwaZulu-Natal: Nongoma, Otobotini, Somkele; Cape Provinces), Sudan, Tanzania, Uganda, Zimbabwe.
African countries where Scirtothrips aurantii has been reported
Occurence of Scirtothrips aurantii in East Africa
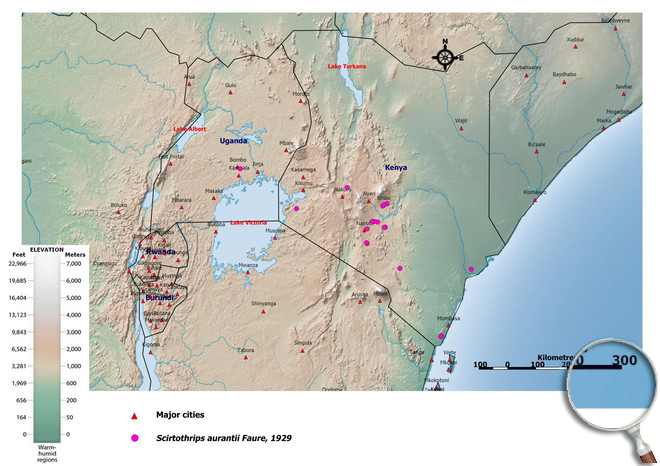
Please click here for survey sites of all observed thrips species of Kenya, Tanzania and Uganda.
Click here for locations of Scirtothrips aurantii in parts of East Africa.

Bibliography
Bailey SF (1964). A revision of the genus Scirtothrips Shull (Thysanoptera: Thripidae). Hilgardia. 35: 329-362
Dennill GB & Erasmus MJ (1992). Basis for a practical technique for monitoring thrips in avocado orchards. Crop Protection. 11 (1): 89-91
El Bashir S & Al Zabidi AH (1985). Fruits spotting of banana in the Yemen Arab Republic. FAO Plant Protection Bulletin. 33 (3): 113-118
Faure JC (1929). The South African citrus thrips and five other new species of Scirtothrips Shull. Transvaal University College Bulletin, Pretoria. 18: 1-18
Gahukar RT (2004). Bionomics and management of major thrips species on agricultural crops in Africa. Outlook on Agriculture. 33 (3): 191-199
Gilbert MJ (1990). Relative population levels of citrus thrips Scirtothrips aurantii on commercial citrus and adjacent bush. South African Journal of Zoology. 25 (1): 72-76
Grout TG (1994). The distribution and abundance of phytoseiid mites (Acari: Phytoseiidae) on citrus in southern Africa and their possible value as predators of citrus thrips (Thysanoptera: Thripidae). Experimental & Applied Acarology. 18 (2): 61-71
Grout TG (2005). Biorational control strategies for Scirtothrips aurantii that minimize non-target effects on arboreal and edaphic predatory mites. VIII International Symposium on Thysanoptera and Tospoviruses, California, September 11-15, 2005
Grout TG & Richards GI (1990). The influence of windbreak species on citrus thrips (Thysanoptera: Thripidae) populations and their damage to South African citrus orchards. Journal of the Entomological Society of Southern Africa. 53 (2): 151-157
Grout TG & Richards GI (1990). Monitoring citrus thrips, Scirtothrips aurantii Faure (Thysanoptera, Thripidae), with yellow card traps and the effect of latitude on treatment thresholds. Journal of Applied Entomology. 109 (4): 385-389
Grout TG & Richards GI (1991). Value of pheromone traps for predicting infestations of red scale, Aonidiella aurantii (Maskell) (Hom, Diaspididae), limited by natural enemy activity and insecticides used to control citrus thrips, Scirtothrips aurantii Faure (Thys,Thripidae). Journal of Applied Entomology. 111 (1): 20-27
Grout TG & Richards GI (1992). Euseius addoensis addoensis, an effective predator of citrus thrips, Scirtothrips aurantii, in the eastern Cape Province of South Africa. Experimental & Applied Acarology. 15 (1): 1-13
Grout TG & Richards RI (1992). The dietary-effect of windbreak pollens on longevity and fecundity of a predacious mite Euseius addoensis-addoensis (Acari: Phytoseiidae) found in citrus orchards in South Africa. Bulletin of Entomological Research. 82 (3): 317-320
Grout TG & Stephen PR (1994). Importation of Neoseiulus cucumeris: how will it affect existing thrips control by Euseius addonensis on citrus? Citrus Journal. 4 (2): 22-24
Grout TG & Stephen PR (2005). Use of an inexpensive technique to compare systemic insecticides applied through drip irrigation systems in citrus. African Entomology. 13 (2): 353-358
Grout TG, Stephen PR & la Croix NJS (1996). Citrus thrips (Thysanoptera: Thripidae) in Swaziland develop tolerance to tartar emetic bait. African Entomology. 4 (1): 15-20
Grovè T, Giliomee JH & Pringle KL (2000). Seasonal abundance of different stages of the citrus thrips, Scirtothrips aurantii, on two mango cultivars in South Africa. Phytoparasitica. 28 (1): 43-53
Grovè T, Giliomee JH & Pringle KL (2001). Thrips (Thysanoptera) species associated with mango trees in South Africa. African Entomology. 9 (2): 153-162
Grovè T, Giliomee JH & Pringle KL (2003). The relationship between citrus thrips, Scirtothrips aurantii (Thysanoptera: Thripidae), abundance and fruit size in mango orchards. African Entomology. 11 (1): 39-48
Grovè T & Pringle KL (2000). A sampling system for estimating population levels of the citrus thrips, Scirtothrips aurantii Faure (Thysanoptera: Thripidae), in mango orchards. African Entomology. 8 (2): 223-226
Hill D (1983). Agricultural insect pests of the tropics and their control, (2nd edition). Cambridge University Press, Cambridge, 746 pp
Hoddle MS, Heraty JM, Rugman-Jones PF, Mound LA & Stouthamer R (2008). Relationships among species of Scirtothrips (Thysanoptera: Thripidae, Thripinae) using molecular and morphological data. Annals of the Entomological Society of America. 101 (3): 491-500
Hoddle MS & Mound LA (2003). The genus Scirtothrips in Australia (Insecta, Thysanoptera, Thripidae). Zootaxa. 268: 1-40
Johansen RM & Mojica-Guzmán A (1998). The genus Scirtothrips Shull, 1909 (Thysanoptera: Thripidae, Sericothripini), in Mexico. Folia Entomologica Mexicana. 104: 23-108
Le Lagadec MD & Bruwer IJ (2002). Alternative control measures for thrips on mangoes. SAMGA-Mango Research Journal, 22: 98-103
Moritz G (2006). Thripse. Pflanzensaftsaugende Insekten, Bd. 1, (1. Auflage). Westarp, Hohenwarsleben, 384 pp. ISBN-13: 978 3 89432 891 7
Moritz G, Delker C, Paulsen M, Mound LA, Burgermeister W (2000). Modern methods in thrips-identification and information (Insecta, Thysanoptera). Bulletin OEPP/EPPO (Paris) 30: 591-593
Moritz G, Morris DC & Mound LA (2001). ThripsID - Pest thrips of the world. ACIAR and CSIRO Publishing Collingwood, Victoria, Australia, CDROM ISBN 1 86320 296 X
Moritz G, Mound LA, Morris DC & Goldarazena A (2004). Pest thrips of the world - an identification and information system using molecular and microscopical methods. Centre for Biological Information Technology, University of Queensland, Australia, CDROM ISBN 1 86499 781 8
Moritz G, O'Donnell C & Parrella M (2009). Pest thrips of North America. Centre for Biological Information Technology, University of Queensland, Australia, CDROM ISBN-13: 978 1 86499 940 2
Morris DC & Mound LA (2004). Molecular relationships between populations of South African citrus thrips (Scirtothrips aurantii Faure) in South Africa and Queensland, Australia. Australian Journal of Entomology. 43 (4): 353-358
Moulton D (1930). Thysanoptera from Africa. Annals and Magazine of Natural History, Zoology, Botany and Geology. (Serie 10) 5: 194-207
Mound LA & Kibby G (1998). Thysanoptera: An identification guide, (2nd edition). CAB International, Wallingford and New York, 70 pp
Mound LA & Marullo R (1996). The thrips of Central and South America: An introduction (Insecta: Thysanoptera). Memoirs on Entomology, International, Vol. 6. Associated Publishers, Gainsville, 487 pp
Mound LA & Palmer JM (1981). Identification, distribution and host-plants of the pest species of Scirtothrips (Thysanoptera: Thripidae). Bulletin of Entomological Research. 71: 467-479
Mound LA & zur Strassen R (2001). The genus Scirtothrips (Thysanoptera: Thripidae) in Mexico: a critique of the review by Johansen & Mojica-Guzmán (1998). Folia Entomologica Mexicana. 40: 133-142
OEPP/EPPO (2005). Scirtothrips aurantii, Scirtothrips citri, Scirtothrips dorsalis. Bulletin OEPP/EPPO Bulletin. 35: 353-356
Palmer JM (1990). Identification of the common thrips of Tropical Africa (Thysanoptera, Insecta). Tropical Pest Management. 36 (1): 27-49
Palmer JM, Mound LA & du Heaume GJ (1989). 2. Thysanoptera, 73 pp. In Betts CR [ed.], CIE Guides to insects of importance to man. CAB International, Wallingford, Oxon, UK
Pitkin BR & Mound LA (1973). A catalogue of West African Thysanoptera. Bulletin de ľInstitut Fondamental ďAfrique Noire, Série A. 35 (2): 407-449
Priesner H (1932). Preliminary notes on Scirtothrips in Egypt, with key and catalogue of the Scirtothrips species of the world. Bulletin de la Société Royale Entomologique ďEgypte. 16: 141-155
Rafter MA, Gillions RM & Walter GH (2008). Generalist herbivores in weed biological control - A natural experiment with a reportedly polyphagous thrips. Biological Control. 44 (2): 188-195
Rugman-Jones PF, Hoddle MS, Mound LA & Stouthamer R (2006). Molecular identification key for pest species of Scirtothrips (Thysanoptera: Thripidae). Journal of Economic Entomology. 99 (5): 1813-1819
Samways MJ (1986). Spatial distribution of Scirtothrips aurantii Faure (Thysanoptera, Thripidae) and threshold level for one percent damage on citrus-fruit based on trapping with fluorescent yellow sticky traps. Bulletin of Entomological Research. 76 (4): 649-659
Samways MJ, Tate BA & Murdoch E (1987). Population-levels of adult citrus thrips Scirtothrips aurantii Faure (Thysanoptera, Thripidae) relative to season and fruit-scarring. Journal of Applied Entomology. 104 (4): 372-377
Schmutterer H (1998). Some arthropod pests and a semi-parasitic plant attacking neem (Azadirachta indica) in Kenya. Anzeiger für Schädlingskunde, Pflanzenschutz, Umweltschutz. 71 (2): 36-38
Strofberg FJ (1948). New host of Scirtothrips aurantii (Faure). Journal of the Entomological Society of Southern Africa. 10: 196-197
Timm AE, Stiller M & Frey JE (2008). A molecular identification key for economically important thrips species (Thysanoptera: Thripidae) in southern Africa. African Entomology. 16 (1): 68-75
Triapitsyn SV (2005). Revision of Ceranisus and the related thrips-attacking entedonine genera (Hymenoptera: Eulophidae) of the world. African Invertebrates. 46: 261-315
zur Strassen R (1960). Catalogue of the known species of South African Thysanoptera. Journal of the Entomological Society of Southern Africa. 23 (2): 321-367
----
Web links
Mound´s Thysanoptera pages
Thysanoptera Checklist
ICIPE Thrips survey sites
UNI Halle & Thrips sites
Thrips of California