|
Specimen preparation
Thrips are small, the body and antennae easily distorted when collecting or handling, and variation in body size and colour is considerable in many species. For these reasons thrips species are easy to misidentify, particularly if the specimens are not mounted onto microscope slides in excellent condition. Thrips should be collected into 60% alcohol (stronger alcohols make the legs and antennae difficult to manipulate), and if possible a small amount of acetic acid and glycerine should be added to this alcohol. This mixture, called AGA, helps to digest the body contents and to keep the specimens flexible. It is mixed in the proportions: 10 parts of 60% alcohol; 1 part of glycerine; 1 part of acetic acid. After collection, if any specimens are to be stored in alcohol rather than mounted onto slides, then these specimens should be removed from the AGA into 60% alcohol and kept in a freezer or at low temperatures. They should never be stored in the light.
Slide mounts for rapid identifications
When slide mounting, a thrips individual should be placed onto a cover slip in a drop of Hoyers. With the ventral surface of the insect uppermost the legs, wings and antennae should be spread, and a microscope slide lowered quickly onto the specimen. The slide is then inverted and placed immediately onto a hot plate (or into an oven) at 45 degrees and left to bake and to clear for several hours. Specimens commonly shrink during the first few minutes, but then expand whilst being heated. Slides should be heated for about 3 weeks and then ring-sealed with nail varnish.
Slide mounts for taxonomic research
The objective is to prepare specimens onto slides with their shape and colour retained in a condition as close as possible to the natural, living state but with the body cleared so that surface detail is visible. To reveal fine details of body sculpture and minute setae, most specimens should be macerated gently. A few specimens should be prepared for study without maceration in order to preserve their natural colouration. Specimens can be manipulated with fine micro-pins, mounted in sealing wax on match-sticks. Use a pair of such pins, one straight the other with the apex bent. A simple lifting tool to move specimens from one dish to another can be made from a small loop of fine wire. Alternatively, alcohols can be changed in dishes using an hypodermic needle or fine glass pipette. The most appropriate dishes to use are 'excavated blocks' - glass blocks 15mm high and 40mm square with a median excavation of about 5ml volume, and with a glass lid to prevent evaporation. Specimens should be macerated, cold, in very weak NaOH solution (2%). Slow maceration removes the body contents and causes minimal damage to the body and wings, particularly if the specimens float on the surface with the wings spread. Dark specimens may be left in this weak solution for longer.
|
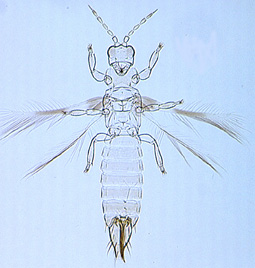
Chaetanaphothrips signipennis |
|
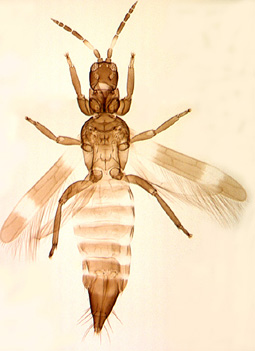
Anisopilothrips venustulus |
|
|
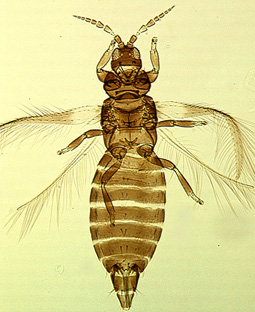
Desmothrips australis |
|
|
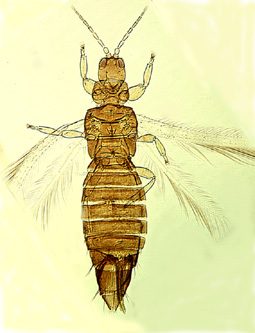
Haplothrips aculeatus |
|
|
Heterothrips arisaemae |
|
|
Holarthrothrips indicus |
|
|
Melanthrips ficalbii |
|