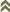Thrips biology, economic importance and control strategies
Thrips biology
The life history of thrips belonging to both suborders Terebrantia and Tubulifera are unique. Eggs are laid either within the plant tissue (Terebrantia) or often on the surface of the plants (Tubulifera). There are two distinct larval stages (Larva I & Larva II) which are vermiform in nature. Both the larval stages move actively and feed on the host plants. The number of prepupal/pupal or resting stages are varied, two in Terebrantia and three in Tubulifera. The pupal stages are found either in the soil or on the plant litter near to the soil and they are inactive non feeding stages. Thrips reproduce by haplodiploidy. The most common form of reproduction is arrhenotoky, where male progeny arises from unfertilized (haploid) eggs and female offspring from fertilized (diploid) eggs. In some species, such as Heliothrips haemorrhoidalis, Hercinothrips bicinctus, Helionothrips errans, Parthenothrips dracaenae, Scirtothrips longipennis, Leucothrips nigripennis, and Chaetanaphothrips orchidii, males are very rare (Lewis 1973). These species reproduce by thelytoky, whereby individual females produce exclusively female progeny by a process that does not involve fertilization. Other species, such as Thrips tabaci or Taeniothrips inconsequens can switch between thely- and arrhenotoky. A screening for the presence of Wolbachia using 16S rDNA and ftsZ gene primers produced interesting results. The arrhenotokous reproducing species Echinothrips americanus and Gynaikothrips ficorum tested positive for Wolbachia, while the bacterium was not detected in Frankliniella occidentalis and in the thelytokous population of Thrips tabaci. Wolbachia was found in the thelytokous reproducing species Hercinothrips femoralis and Parthenothrips dracaenae (Kumm & Moritz 2008).
Fig. 1: Thrips ontogenesis and tospovirus replication and transmission cycle
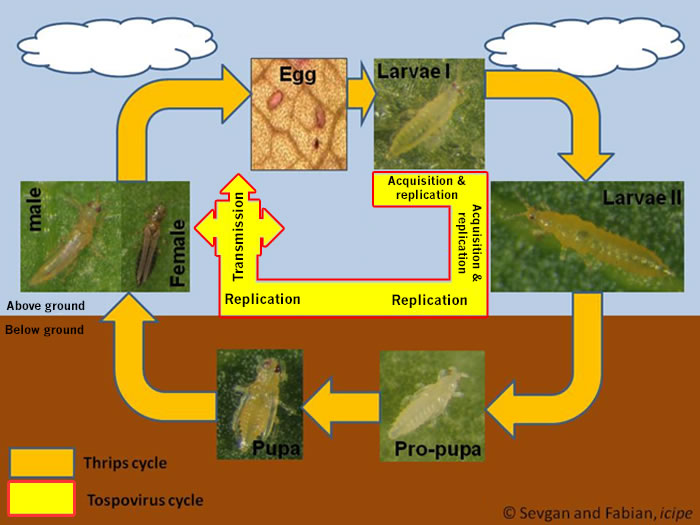
The ability of thrips to transmit tospoviruses is subject of a dynamic process and depends on the vector status and the genetic determinants of the tospovirus (Sin et al. 2005; Ullman et al. 2005). Presently there are about 11 species of more than 6000 thrips known to vector Tospoviruses to a wide range of plants belonging to more than 1000 species in about 90 families (German et al. 1992; Mound 1996; Ullman 1996) (Fig. 1 and Tab. 1). Only adult thrips that have acquired a virus during their first or early second larval stage can transmit tospoviruses (van de Wetering et al. 1996; Amin et al. 1981, German et al. 1992, Ullman et al. 1991, 1992, 1995). Usually high titers of viruses can be measured within the midgut epithel cells, the visceral muscle cells of the anterior midgut region, the lobed salivary glands and the malpighian tubules, but not within the haemocoel (Fig.2). Such results emphasis the point made earlier that virus particles must move from the visceral muscle tissue to the membrane of the lobed salivary gland cells to avoid the hemocoel. This is only possible during a very short time window of a temporary association of the cell membranes of midgut epithel cells, visceral muscle cells and cells of the lobed salivary gland during the first larval stage. This complex is the result of displacement of the brain into the prothoracic region by enlarged cibarial muscle groups. However, the examinations of morphogenetic movements during ontogenic development has provided a totally new view of the likely virus pathway through a vector (Moritz et al. 2004).
Apart from the tospoviruses, thrips species like Frankliniella williamsi are known to vector Machlomovirus like maize chlorotic mottle machlomovirus (MCMV) in Poaceaous crops such as Maize. Unlike with the tospoviruses, where transmission is circulative and persistant, MCMV is transmitted in a non-persistent manner. Some populations of typical vector species, such as Thrips tabaci, are known to have lost their ability to transmit, whereas populations of other species may increase their vector potential. Such phenomenon needs to be considered in future research efforts to undestand factors influencing thrips-tospovirus interaction, such as host plants, geographic area, climatic conditions, population and reproduction status of the vector etc.
Tab. 1: Tospovirus-Vectors, origin and transmitted tospoviruses (CaCV: Capsicum chlorosis virus, GRSV: Groundnut ringspot virus, INSV: Impatiens necrotic spot virus, TCSV: Tomato chlorotic spot virus, TSWV: Tomato spotted wilt virus, WSMV: Watermelon silver mottle tospovirus, CSNV: Chrysanthemum stem necrosis virus, IYSV: Iris yellow spot virus, TYRV: Tomato yellow ring virus, GBNV: Groundnut bud necrosis virus, CLCSV: Calla lily chlorotic spot virus, MYSV: Melon yellow spot virus, PCFV: Peanut chlorotic fan-spot virus, PYSV: Peanut yellow spot virus, ZLCV: Zucchini lethal chlorosis virus, PRSV: Polygonum ring spot virus) (Riley et al. 2011)
| virus vector | origin | virus | genus |
|---|---|---|---|
| Frankliniella fusca | Eastern USA | TSWV, INSV, IYSV | 8 of 230 species of the genus Frankliniella |
| Frankliniella intonsa | Europe to Asia | TSWV, TCSV, GRSV, INSV | |
| Frankliniella occidentalis | Western USA | TSWV, INSV, GRSV, TCSV, CSNV | |
| Frankliniella schultzei | South America | TSWV, TSCV, GRSV, GBNV, CSNV | |
| Frankliniella zucchini | South America | ZLCV | |
| Frankliniella cephalica | Central America | TSWV | |
| Frankliniella gemina | South America | TSWV, GRSV | |
| Frankliniella bispinosa | South East USA | TSWV (?) | |
| Thrips palmi | South East Asia | CLCSV, GBNV, MYSV, TSWV, WSMV | 3 of 286 species of the genus Thrips |
| Thrips setosus | Japan | TSWV | |
| Thrips tabaci | Eastern Mediterranean | TSWV, IYSV, TYRV | |
| Scirtothrips dorsalis | South East Asia | GBNV, PCFV, PYSV | 1 of 102 |
| Ceratothripoides claratris | South East Asia | CaCV(+/-) | 1 of 5 |
| Dictyothrips betae | Central Europe | PRSV | 1 of 1 |
Fig. 2: Frankliniella occidentalis: Larval anatomy with a closely contact between salivary gland and anterior part of the midgut.
Bibliography and important links to thrips and tospoviruses
Amin PW, Reddy DVR, Ghanekar AM & Reddy MS (1981). Transmission of Tomato Spotted Wilt Virus, the causal agent of bud necrosis of peanut, by Scirtothrips dorsalis and Frankliniella schultzei. Plant Disease 65 (8): 663-665
Birithia R, Subramanian S, Pappu HR, Sseruwagi P, Muthomi JW & Narla RD (2011). First report of Iris Yellow spot virus infecting onion in Kenya and Uganda. Plant Disease 95: 1195
Birithia R, Subramanian S, Villinger J, Muthomi JW, Narla RD & Pappu HR (2012). First report of Tomato yellow ring virus (Tospovirus, Bunyaviridae) infecting tomato in Kenya. Plant Disease 96: 1384
Brittlebank, CC (1919). Tomato diseases. J Agric Vicotria 17: 231-235
Dugje IY, Omoigui LO, Ekeleme F, Kamara AY & Ajeigbe H (2009). Farmers- Guide to Cowpea Production in West Africa. IITA, Ibadan, Nigeria. 20 pp
German TL, Ullman DE, Moyer JW (1992). Tospoviruses: Diagnosis, molecular biology, phylogeny, and vector relationships. Annu Rev Phytopathol 30: 315–348
Jackai LEN & Daoust RA (1986). Insect Pests of Cowpeas. Annual Review of Entomology 31:95-119.
Jones DR (2005). Plant viruses transmitted by thrips. European Journal of Plant Pathology 113: 119-157
Milne, RG, Francki RI (1984). Should tomato spotted wilt virus be considered as a possible member of the family Bunyaviridae? Intervirology 22: 72-76
Moritz G, Kumm S, Mound LA (2004). Tospovirus transmission depends on thrips ontogeny. Virus Research 100: 143-149
Mound LA (1996). The Thysanoptera vector species of Tospoviruses. Acta Horticulturae 431: 298-309
Nagata T, Inoue-Nagata AK, Smid HM, Goldbach R, Peters D (1999). Tissue tropism related to vector competence of Frankliniella occidentalis for tomato spotted wilt tospovirus. J Gen Virol 80 (Pt 2): 507-515
Nyasani JO, Meyhöfer R, Subramanian S & Poehling H-M (2012). Effect of intercrops on thrips species composition and population abundance on French beans in Kenya. Entomologia Experimentalis et Applicata 142: 236 - 246
Pittman, HA (1927). Spotted wilt of tomatoes. Prelimary note concerning the transmission of the "spotted wilt" of tomatoes by an insect vector (Thrips tabaci Lind.). Aust J Counc Sci Ind Res 1: 74-77
Ramkat RC, Wangai AW, Rapando PN & Lelgut DK (2008). Cropping system influences Tomato spotted wilt virus disease development, thrips population and yield of tomato (Lycoperscion escullentum). Journal of Applied Biology, 153, 373-380
Rapando P, Wangai AW, Tabu I & Ramkat R (2009). Variety, mulch and stage of inoculation effects on incidence of tomato spotted wilt virus disease in cucumber (Cucumis sativus L.) Archives of Phytopathology and Plant Protection 42: 579 - 586
Riley DG, Joseph SV, Srinivasan R & Diffe S (2011). Thrips vectors of tospoviruses. Journal of Integrated Pest Management 1(2): DOI:10.1603/IPM10020
Samuel G, Bald JG, Pittman HA (1930). Investigations on "spotted wilt" of tomatoes. Australia Commonw Counc Sci Ind Res Bull No. 44
Schreiter G, Butt TM, Beckett A, Vestergaard S, Moritz G (1994). Invasion and development of Verticillium lecanii in the western flower thrips Frankliniella occidentalis. Mycol. Res. 98(9): 1025-1034
Sin S-H, McNulty B, Kennedy GG, Moyer, JW (2005). Viral genetic determinants for thrips transmission of Tomato spotted wilt virus. PNAS 102, 5168-5173
Ullman DE (1996). Thrips and Tospoviruses: Advances and future directions. Acta Horticulturae 431: 310-323
Ullman DE, Cho JJ, Mau RFL, Hunter WB, Westcot DM, Custer DM (1992): Thrips-Tomato Spotted Wilt Virus interactions: Morphological, behavioral and cellular components influencing thrips transmission. Advances in Disease Vector Research 9: 195-240
Ullman DE, Meideros R, Campbell LR, Whitfield AE, Sherwood JL, German TL (2002). Thrips as vectors of tospoviruses. Advances in Botanical Research 36:113-140
Ullman DE, Whitfield AE, German TL (2005). Thrips and tospoviruses come of age: Mapping determinants of insect transmission. PNAS 102: 4931-4932
Van de Wetering F, Goldbach R, Peters D (1996). Tomato spotted wilt tospovirus ingestion by first instar larvae of Frankliniella occidentalis is a prerequisite for transmission. Phytopathology 86: 900-905
Wangi AW, Mandal B, Pappu HR & Kilonzo S (2001). Outbreak of tomatoes spotted wilt virus in tomato in Kenya. Plant Diseases, 85: 1123
Whitfield AE, Ullmann DE, German TL (2005). Tospovirus-Thrips interactions. Anny Rev Phytopathol 43: 459-489
----
Web links
Tomato spotted wilt virus
Mound´s Thysanoptera pages
Thysanoptera Checklist
ICIPE Thrips survey sites
UNI Halle & Thrips sites
Thrips of California