History
The disease "spotted wilt" was first described
in Australia (Brittlebank
1919). Nearly ten years later Pittman (1927) gave a prelimary note concerning
the transmission of this disease of tomatoes by Thrips
tabaci. Samuel et al. (1930) could prove that the disease
is caused by a virus, which they named TSWV (Tomato spotted wilt virus).
Milne & Francki
(1984) noted similarities between TSWV and viruses of the family Bunyaviridae
(Orthobunyavirus, Hantavirus, Nairovirus, Phlebovirus), an animal-infecting
taxon with tripartite negative- and ambisense stranded RNA genomes. Molecular
data of the last decades support the taxonomic position of Tospoviruses
as a first group of Phytoarbovirus members of the Bunyaviridae (see summary
in German et al. 1992, Jones 2005, Whitfield et al. 2005).
Virus-Vector Interactions
The ability of thrips to transmit tospoviruses is subject of a dynamic process and depends on the vector status and the genetic determinants of the tospovirus (Sin et al. 2005, Ullman et al. 2005). There are now about 11 species of thrips known to carry Tospoviruses to a wide range of plants of more than 500 species in more than 50 families (German et al. 1992; Mound 1996; Ullman 1996) (Tab. 1). Only adult thrips that have acquired a virus during their first or early second larval stage can transmit tospoviruses (van de Wetering et al. 1996; Amin et al. 1981, German et al. 1992, Ullman et al. 1991, 1992, 1995). Some populations of typical vector species, such as Thrips tabaci, are known to have lost their ability to transmit, whereas populations of other species may increase their vector potential. This in mind it is important for future research to make notes of the circumstances of a thrips-tospovirus-liaison, like host plants, geographic area and climatic conditions, population and reproduction status of the vector etc. For many years, it has been known that second stage larvae and adult thrips can only transmit tospoviruses if virus acquisition occurred whilst first larvae fed on infected plants (van de Wetering et al. 1996) (Fig. 1).
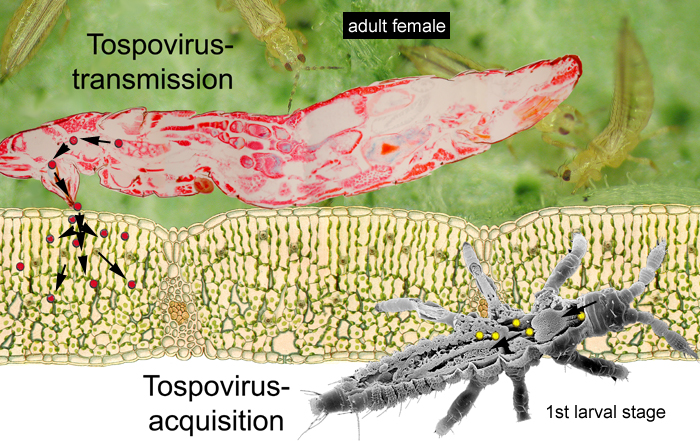
Fig. 1: Frankliniella occidentalis: Larval acquisition and adult transmission of TSWV (© GMoritz)
The general pathway through the arthropod vector is similar for all these viruses with one exception in thrips, the hemocoel. The competence to transmit tospoviruses successful is determined by the ability of the virus to infect vector tissues and by the ability of the virus to exit tissue barriers (Ullman et al. 1992). Usually high titers of viruses can be measure within the midgut epithel cells, the visceral muscle cells of the anterior midgut region, the lobed salivary glands and the malpighian tubules, but not within the mixocoel (Fig.2).
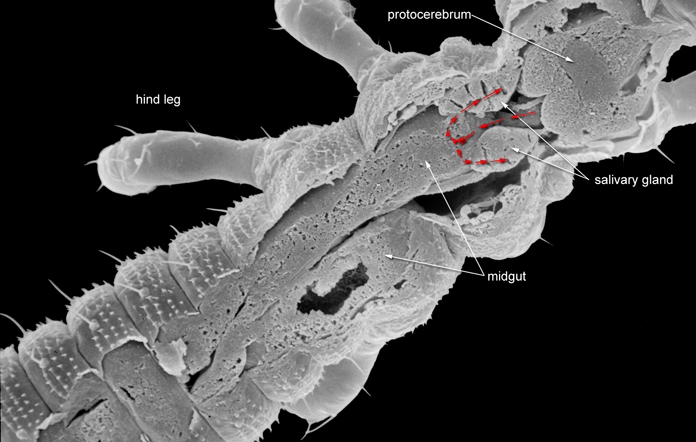
Fig. 2: Frankliniella occidentalis: Larval I anatomy showing a close relation between anterior part of the midgut and lobed salivary glands (© GMoritz)
Such results emphasis the point made earlier that virus particles must make a jump from the visceral muscletissue to the membrane of the lobed salivary gland cells to avoid the hemocoel. This is only possible in a very short time window of a temporary association of the cell membranes of midgut epithel cells, visceral muscle cells and cells of the lobed salivary gland during the first larval stage. This complex is the result of displacement of the brain into the prothoracic region by enlarged cibarial muscle groups. However, these examinations of morphogenetic movements during ontogenetic development has provided a totally new view of the likely virus pathway through a vector (Moritz et al. 2004). The loss of the fusion and tight contact of salivary and midgut cells via visceral muscle cells leads to a strong input of virus particles into the haemocoel. From there, these particles are presumably transported to the malpighian tubules and the fluorescence signals in these tubules give the impression of further replication in those cells. In contrast Nagata et al. (1999) described and discussed a hollow paired ligament that they consider functions as a virus canal between the midgut and the salivary gland. Anatomically such ligaments fixes the germaria of the paired four ovarioles within the thoracic endoskeleton (Moritz 1989). The continued presence of this structure contrasts with the limited acquisition period demonstrated here.
Tab. 1: Tospovirus-Vectors, origin and transmitted tospovirus members
| virus vector | origin | virus | genus |
|---|---|---|---|
| Frankliniella fusca | eastern USA | TSWV | 5 to 6 of 160species of the genus Frankliniella |
| Frankliniella intonsa | Europe to Asia | TSWV, TCSV, GRSV | |
| Frankliniella occidentalis | Western USA | TSWV, INSV, GRSV, TCSV | |
| Frankliniella schultzei | S. America | TSWV, TSCV, GRSV | |
| Frankliniella zuchini | S. America | (?) | |
| Frankliniella bispinosa | S.E. USA | TSWV (?) | |
| Thrips palmi | S.E. Asia | WSMV, GBMV | 3 of 280 species of the genus Thrips |
| Thrips setosus | Japan | TSWV | |
| Thrips tabaci | E. Mediterranean | TSWV | |
| Scirtothrips dorsalis | S.E. Asia | TSWV | 1 of 90 |
| Ceratothripoides claratris | S.E. Asia | CaCV | 1 of 9 |
| INSV: impatiens necrotic spot tospovirus | TSWV: tomato spotted wilt (tospo)virus | ||
| GRSV: groundnut ringspot (tospo)virus | WSMV: watermelon silver mottie tospovirus | ||
| TCSV: tomato chlorotic spot tospovirus | CaCV: capsicum chlorosis virus | ||
References to Thrips and Tospoviruses
Amin PW; Reddy DVR; Ghanekar AM (1981): Transmission of tomato spotted wilt virus, causal agent of bud encrosis of peanut, by Scirtothrips dorsalis and Frankliniella schultzei. Plant Disease 65: 663-665.
Brittlebank, CC (1919): Tomato diseases. J Agric Vicotria 17: 231-235.
German TL; Ullman DE; Moyer JW (1992): Tospoviruses: Diagnosis, molecular biology, phylogeny, and vector relationships. Annu Rev Phytopathol 30: 315–348.
Jones DR (2005): Plant viruses transmitted by thrips. European Journal of Plant Pathology 113: 119-157.
Milne, RG; Francki RI (1984): Should tomato spotted wilt virus be considered as a possible member of the family Bunyaviridae? Intervirology 22: 72-76.
Pittman, HA (1927): Spotted wilt of tomatoes. Prelimary note concerning the transmission of the "spotted wilt" of tomatoes by an insect vector (Thrips tabaci Lind.). Aust J Counc Sci Ind Res 1: 74-77.
Moritz G; Kumm S; Mound LA (2004): Tospovirus transmission depends on thrips ontogeny. Virus Research 100: 143-149.
Moritz G (1989): Die Ontogenese der Thysanoptera (Insecta) unter besonderer Berücksichtigung des Fransenflüglers Hercinothrips femoralis (O.M.REUTER, 1891) (Thys., Thripidae, Panchaetothripinae) - 5. Mitt. Imago - Thorax. Zool Jb Anat 118: 391-427.
Mound LA (1996):The Thysanoptera vector species of Tospoviruses. Acta Horticulturae 431: 298-309.
Nagata T; Inoue-Nagata AK; Smid HM; Goldbach R; Peters D (1999): Tissue tropism related to vector competence of Frankliniella occidentalis for tomato spotted wilt tospovirus. J Gen Virol 80 (Pt 2): 507-15.
Samuel, G; Bald, JG; Pittman, HA (1930): Investigations on "spotted wilt" of tomatoes. Australia Commonw Counc Sci Ind Res Bull No. 44.
Sin S-H; McNulty B; Kennedy GG; Moyer, JW (2005): Viral genetic determinants for thrips transmission of Tomato spotted wilt virus. PNAS 102, 5168-5173.
Ullman DE (1996): Thrips and Tospoviruses: Advances and future directions. Acta Horticulturae 431: 310-323.
Ullman DE; Cho JJ; Mau RFL; Hunter WB; Westcot DM; Custer DM (1992): Thrips-Tomato Spotted Wilt Virus interactions: Morphological, behavioral and cellular components influencing thrips transmission. Advances in Disease Vector Research 9: 195-240.
Ullman DE; Whitfield AE; German TL (2005): Thrips and tospoviruses come of age: Mapping determinants of insect transmission. PNAS 102: 4931-4932.
Van de Wetering F; Goldbach R; Peters D (1996): Tomato spotted wilt tospovirus ingestion by first instar larvae of Frankliniella occidentalis is a prerequisite for transmission. Phytopathology 86: 900-905.
Whitfield AE; Ullmann DE; German TL (2005): Tospovirus-Thrips interactions. Anny Rev Phytopathol 43: 459-489.Complete Tospovirus Resource Page - Kansas State University & University of California