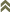Figures
Fig.†1: Antenna (inset: III. and†IV. antennal segment)
Fig. 2: Head dorsal with ocellar triangle
Fig. 3: Pronotum
Fig. 4: Meso- and metanotum
Fig. 5: Fore- and hindwing
Fig. 6: Sternite VII
Fig. 7: Tergites V and VI
Fig. 8:†Tergites VIII-X
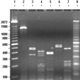
ITS-RFLP gel patterns (1&8 ladder, 2 PCR-product, 3 RSAI, 4 HaeIII, 5 MspI, 6 HinfI, 7 AluI)
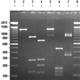
Fig. 9: Primer pair O1/18J
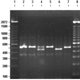
Fig. 10: Primer pair P1/28Z
Fig. 11: Primer pair TODA1/TODA2
Taxonomic Information
Species:
Thrips obscuratus (Crawford JC, 1941)
Synonyms:
Isoneurothrips obscuratus Crawford JC, 1941
Common name:
New Zealand flower thrips
Present taxonomic position:
Family: Thripidae Stephens, 1829
Subfamily: Thripinae (Stephens) Karny, 1921
Genus: Thrips Linneaeus, 1758
Species Recognition
General information about the genus Thrips:There are about 280 species currently recognized in the genus Thrips making this genus one of the largest groups within the Thysanoptera. They are separated from other genera in having the following characters, antenna comprising 7 or†8 segments with segments III and IV containing forked sense cones, the head has two pairs of ocellar setae (II and III), pair I is missing, the pronotum with four long setae on the posterior margin, forewing 1st vein usually has a row of setae interrupted by gaps, on lateral sides of abdominal tergites V to VIII there are paired ctenidia, abdominal tergite VIII with ctenidia posterior to†the spiracles.
Typical character states of Thrips obscuratus:
Body color
Mainly brown or pale or yellow,†with some darker markings
Antennae
Number of antennal segments: 7-8
Segment IV - forked sensorium:†scarcely extending beyond base of segment V
Segments II and III shape:†more or less symmetric
Segments III & IV sensoria:†emergent and forked
Base of sensorium on antennal segment VI:†no more than 2 times as wide as base of nearest seta
Terminal antennal segments:†rarely elongate
Head:
Distance between bases of ocellar setae III:†greater than width of first ocellus
Head shape between compound eyes:†not prolonged
Ocellar setae III on head:†arising on anterior margin of, or in front of, ocellar triangle
Postocular setae I:†absent
Surface of head, pronotum and fore legs:†without strong reticulate sculpture
Ocellar setae I in front of anterior ocellus:†absent
Prothorax
Number of pairs of elongate pronotal setae: 0-3
Number of pairs of elongate posteroangular pronotal setae: 2
Pronotum shape:†rectangular
Number of pairs of pronotum posteromarginal minor setae: 2-4
Number of pairs of pronotum anteromarginal minor setae: 3-4
Mesothorax
Mesothoracic endofurca:†with median spinula
Metathorax
Metanotal median area sculptured lines: transverse at anterior, but longitudinal and parallel on posterior half
Metanotal median setae length:†longer than lateral metanotal setae
Metanotal median setae position:†arising at anterior margin
Metanotum:†without campaniform sensilla
Metanotum major sclerite:†with two major sclerites, metascutum and metascutellum
Metanotum median area:†with no equiangular reticulation
Metanotum sculpture:†without dominant sculptured triangle medially
Metathoracic endofurca:†transverse, sometimes with simple median spinula
Wings
Wings:†present and more than half as long as abdomen
First vein of forewing:†distinct from costal vein
Forewing anterior margin:†with setae and cilia but cilia longer than setae
Forewing clavus:†terminal veinal seta longer than subterminal seta
Forewing color:†uniformly dark or shaded, but with base (or sub-base) pale or uniformly light brown
Forewing costal fringe of cilia:†arising at anterior margin of wing
Forewing costal setae at middle of wing:†shorter than median width of wing
Forewing first vein setal row:†complete, with setae closely and uniformly spaced
Forewing posterior margin cilia:†undulated near apex
Forewing second vein setal row:†complete, with setae closely and uniformly spaced
Forewing surface:†not reticulate
Forewings:†with veins, setae and microtrichia
Legs
Fore tarsus inner apex:†without tooth
Fore tibial apex:†not extending around fore tarsus -†with small curved claw ventrolaterally
Mid and hind tarsi:†with two segments
Abdomen:
Pleurotergal discal setae:†present
Abdominal pleurotergites:†not covered in microtrichia
Abdominal segment X:†never tubular, longitudinally incomplete ventrally in both sexes
Abdominal sternite II:†with 1 or 2 discal setae in addition to marginal setae
Abdominal sternite III of female:†without glandular areas
Abdominal sternite VII:†with discal setae present on median area
Abdominal sternite VII median marginal setae: arising in front of margin
Abdominal sternites IV , V and VI:†with discal setae present medially as well as marginal setae
Number of lateral marginal setae on abdominal tergite II: 3
Abdominal tergites:†without curved wing-retaining setae
Abdominal tergites IV & V median setal pair:†much shorter than distance between their bases
Abdominal tergites V-VII:†with pair of ctenidia laterally
Number of discal setae on sternite V: 3-13
Setae on abdominal tergite X:†slender
Surface of lateral thirds of abdominal tergites:†without regular rows of fine microtrichia
Ctenidia on tergite VIII:†posteromesad to spiracle
Tergite VIII posteromarginal comb of microtrichia:†present, complete medially
Tergite VIII posteromarginal microtrichia:†long, slender and irregular
Biology
Life history:
As with other thrips species the life cycle from egg to adult is dependent on†temperature.†The full cycle can take about 15 days (Lewis, 1973) to over a month and adults may live for more than one month producing several generations in one year depending on seasonal weather. With greenhouse temperatures the developmental time from egg to adult can decrease to about one week.
Host plants:
Polyphagous
Vector capacity:
Can carry spores of Monilinia fructicola causing brown rot on stone fruit.
Current known distribution:
Australia, New Zealand
Additional notes:
While this species is known to be exported on pome fruits itís currently restricted to Australia and New Zealand and feeds on a wide range of host plants.
Bibliography
Bailey, SF (1957): The thrips of
California Part I: Suborder Terebrantia. Bulletin of the California Insect
Survey 4, no. 5: 143-220.
Berry, N, Butler, RC & Teulon, DAJ (2006): Responses of New Zealand flower thrips (Thrips obscuratus) to synthetic and natural stimuli (odour and colour) in a wind tunnel. New Zealand Journal of Crop and Horticultural Science, 34, 121-129.
Blank, RH & Gill, GSC (1997): Thrips (Thysanoptera : Terebrantia) on flowers and fruit of citrus in New Zealand. New Zealand Journal of Crop and Horticultural Science, 25, 319-332.
Carpenter, A, Wright, S & Lash, P (1996): Response of adult New Zealand flower thrips, Thrips obscuratus (Thysanoptera: Thripidae) to high carbon dioxide and low oxygen atmospheres at various temperatures. Bulletin of Entomological Research, 86, 217-221.
Davidsonz, MM, Perry, NB, Larsen, L, Green, VC, Butler, RC & Teulon, DAJ (2008): 4-pyridyl carbonyl compounds as thrips lures: Effectiveness for western flower thrips in Y-tube bioassays. Journal of Agricultural and Food Chemistry, 56, 6554-6561.
Dymock, JJ & Holder, PW (1996): Nationwide survey of arthropods and molluscs on cut flowers in New Zealand. New Zealand Journal of Crop and Horticultural Science, 24, 249-257.
Fermaud, M & Gaunt, RE (1995): Thrips obscuratus as a Potential Vector of Botrytis cinerea in Kiwifruit. Mycological Research, 99, 267-273.
Fermaud, M, Gaunt, RE & Elmer, PAG (1994): The Influence of Thrips obscuratus on Infection and Contamination of Kiwifruit by Botrytis cinerea. Plant Pathology, 43, 953-960.
Lewis, T (1973): Thrips their biology, ecology and economic
importance. Academic Press Inc., London Ltd. 349 pp.
Liu, YB (2003): Effects of vacuum and controlled atmosphere treatments on insect mortality and lettuce quality. Journal of Economic Entomology, 96, 1100-1107.
Michailides, TJ & Elmer, PAG (2000): Botrytis gray mold of kiwifruit caused by Botrytis cinerea in the United States and New Zealand. Plant Disease, 84, 208-223.
Miyazaki, M & Kudo,
I (1987): Occurrence of the Gladiolus Thrips, Thrips
simplex (Morison), in Japan (Thysanoptera, Thripidae). Applied Entomology and
Zoology, 22, 230-232.
Moritz G, Morris DC, Mound LA (2001): ThripsID -
Pest thrips of the world. ACIAR
and CSIRO Publishing Collingwood, Victoria, Australia, CDROM ISBN 1
86320 296 X.
Moritz G, Mound LA, Morris DC, Goldarazena A (2004): Pest
thrips of the world - an identification and information system using molecular
and microscopial methods. CBIT, University of Queensland,CDROM ISBN 1-86499-781-8.
Mound, LA (2005): The Thrips orientalis group from South-east Asia and Australia: some species identities and relationships (Thysanoptera, Thripidae). Australian Journal of Entomology, 44, 420-424.
Mound,
LA & Kibby, G (1998): Thysanoptera: An identification guide, (2nd
edition). CAB International, Wallingford and New York, 70pp.
Mound, LA & Marullo, R (1996): The
thrips of Central and South America: An Introduction (Insecta: Thysanoptera).
Associated Publishers, Gainesville.
Mound, LA & Masumoto, M (2005): The genus Thrips (Thysanoptera, Thripidae) in Australia, New Caledonia and New Zealand. Zootaxa, 3-64.
Nakahara, S (1985): Review of Thrips hawaiiensis and revalidation of T. florum (Thysanoptera: Thripidae). Proceedings of the Entomological Society of Washington, 87:864-870.
Nakahara, S (1994): The Genus Thrips Linnaeus
(Thysanoptera: Thripidae) of the New World. USDA Agricultural Research
Service Technical bulletin No. 1822.
Penman, DR, Osborne, GO, Worner, SP, Chapman, RB & Mclaren, GF (1982): Ethyl Nicotinate - a chemical attractant for Thrips obscuratus (Thysanoptera, Thripidae) in stonefruit in New-Zealand. Journal of Chemical Ecology, 8, 1299-1303.
Potter, MA, Carpenter, A, Stocker, A & Wright, S (1994): Controlled atmospheres for the postharvest disinfestation of Thrips obscuratus (Thysanoptera, Thripidae). Journal of Economic Entomology, 87, 1251-1255.
Schmidt, K, Wratten, SD, Teulon, DAJ, Jaspers, MV & Beever, RE (2007): Population dynamics of the New Zealand flower thrips (Thrips obscuratus) and possible consequences for the incidence of Botrytis bunch rot in grapes. Journal of Insect Science, 7.
Stannard, LJ (1968): The thrips, or Thysanoptera, of
Illinois. Illinois Natural History Survey Bulletin 29: 215-552.
Teulon, DAJ, Butler, RC, James, DE & Davidson, MM (2007): Odour-baited traps influence thrips capture in proximal unbalted traps in the field. Entomologia Experimentalis Et Applicata, 123, 253-262.
Teulon, DAJ, Davidson, MM, Hedderley, DI, James, DE, Fletcher, CD, Larsen, L, et al. (2007): 4-pyridyl carbonyl and related compounds as thrips lures: Effectiveness for onion thrips and new zealand flower thrips in field experiments. Journal of Agricultural and Food Chemistry, 55, 6198-6205.
Teulon, DAJ & Penman, DR (1990): Host records for the New Zealand flower thrips (Thrips obscuratus (Crawford) Thysanoptera: Thripidae). New Zealand Entomologist 13: 46-51.
Teulon, DAJ & Penman, DR (1991): Effects of temperature and diet on oviposition rate and development time of the New-Zealand Flower Thrips, Thrips obscuratus. Entomologia Experimentalis et Applicata, 60, 143-155.
Teulon, DAJ & Penman, DR (1996): Thrips (Thysanoptera) seasonal flight activity and infestation of ripe stonefruit in Canterbury, New Zealand. Journal of Economic Entomology, 89, 722-734.
Teulon, DAJ, Penman, DR & Ramakers, PMJ (1993): Volatile chemicals for thrips (Thysanoptera, Thripidae) host-finding and applications for thrips pest-management. Journal of Economic Entomology, 86, 1405-1415.
Wearing, CH & Colhoun, K (1999): Development of Orius vicinus (Ribaut) (Heteroptera : Anthocoridae) on different prey. Biocontrol Science and Technology, 9, 327-334.
Links:
Mound, LA (2005): Thysanoptera (Thrips) of the World
- A Checklist. http://www.ento.csiro.au/thysanoptera/worldthrips.html